Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer
- PMID: 37275963
- PMCID: PMC10233570
- DOI: 10.1177/17588359231178125
Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer
Erratum in
-
Corrigendum to Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer.Ther Adv Med Oncol. 2023 Sep 29;15:17588359231201818. doi: 10.1177/17588359231201818. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37786535 Free PMC article.
Abstract
Background: Ribociclib has demonstrated a statistically significant overall survival benefit in pre- and postmenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2-) advanced breast cancer. New Adjuvant Trial with Ribociclib [LEE011] (NATALEE) is a trial evaluating the efficacy and safety of adjuvant ribociclib plus endocrine therapy (ET) versus ET alone in patients with HR+/HER2- early nonmetastatic breast cancer (EBC).
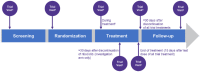
Methods/design: NATALEE is a multicenter, randomized, open-label, Phase III trial in patients with HR+/HER2- EBC. Eligible patients include women, regardless of menopausal status, and men aged ⩾18 years. Select patients with stage IIA, stage IIB, or stage III disease (per the anatomic classification in the AJCC Cancer Staging Manual, 8th edition) with an initial diagnosis ⩽18 months prior to randomization are eligible. Patients receiving standard (neo)adjuvant ET are eligible if treatment was initiated ⩽12 months before randomization. Patients undergo 1:1 randomization to ribociclib 400 mg/day (3 weeks on/1 week off) +ET (letrozole 2.5 mg/day or anastrozole 1 mg/day [investigator's discretion] plus goserelin [men or premenopausal women]) or ET alone. Ribociclib treatment duration is 36 months; ET treatment duration is ⩾60 months. The primary end point is invasive disease-free survival.
Discussion: The 36-month treatment duration of ribociclib in NATALEE is extended compared with that in other adjuvant cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor trials and is intended to maximize efficacy due to longer duration of CDK4/6 inhibition. Compared with the 600-mg/day dose used in advanced breast cancer, the reduced ribociclib dose used in NATALEE may improve tolerability while maintaining efficacy. NATALEE includes the broadest population of patients with HR+/HER2- EBC of any Phase III trial currently evaluating adjuvant CDK4/6 inhibitor treatment.
Trial registration: ClinicalTrials.gov identifier: NCT03701334 (https://clinicaltrials.gov/ct2/show/NCT03701334).
Keywords: CDK4/6 inhibitors; HR+/HER2– early breast cancer; NATALEE; adjuvant therapy; ribociclib.
© The Author(s), 2023.
Conflict of interest statement
Dr. Slamon reports stock ownership from BioMarin, Pfizer, Amgen, Seattle Genetics, TORL BioTherapeutics, 1200 Pharma; travel support from BioMarin, Pfizer, Novartis; personal fees from Novartis, Eli Lilly; grants from Pfizer, Novartis; founder of 1200 Pharma, TORL BioTherapeutics. Dr. Fasching reports personal fees from Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, Eisai, MSD, Lilly, Pierre Fabre, Seagen, F. Hoffmann-La Roche, Hexal, Agendia, Sanofi, Gilead; institutional funding from BioNTech, Pfizer, Cepheid; research grant from Pfizer. Dr. Hurvitz has nothing to disclose. Dr. Chia reports personal fees and grants to institution from Novartis, Pfizer, F. Hoffmann-La Roche, Eli Lilly. Prof. Crown has nothing to disclose. Dr. Martín reports personal fees from Lilly, Pfizer, AstraZeneca, Novartis, Roche/Genentech, GSK, PharmaMar, Taiho Oncology; research grants from Novartis, Roche-Genentech. Dr. Barrios reports institutional research grants from Pfizer, PharmaMar, Polyphor, Henlius Biotech, Shanghai, Merck KGaA, Millennium, LEO Pharma, ImClone Systems, Exelixis, Medivation, Asana Biosciences, AB Science, Abraxis Biosciences, Daiichi Sankyo, Bristol Myers Squibb, BioMarin, Astellas Pharma, AbbVie, Merck (MSD), Merrimack, Mylan, Taiho Pharmaceutical, Sanofi, GSK, Roche/Genentech, Lilly, Boehringer Ingelheim, Novartis, AstraZeneca, Amgen, Pfizer; personal fees from Boehringer Ingelheim, Sanofi, Lilly, Zodiac, AstraZeneca, MSD, Bayer, Eisai, Roche/Genentech, Pfizer, Novartis, GSK, Daiichi Sankyo; stock from MEDSIR, Thummi. Dr. Bardia reports research grants to institution from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics, Mersana, Innocrin; personal fees from Biothermostics, Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Spectrum Pharma, Taiho, Sanofi, Daiichi Sankyo, Puma. Dr. Im reports personal fees from AstraZeneca, Novartis, Hanmi, Pfizer, Eisai, Amgen, Roche, Lilly, GSK, MSD; research grants from AstraZeneca, Pfizer, Eisai, F. Hoffmann-La Roche, Daewoong Pharmaceuticals. Dr. Yardley reports research funding to institution from Daiichi Sankyo/Lilly, Eisai, Roche/Genentech, Novartis, AbbVie, AstraZeneca, Clovis Oncology, Immunomedics, InventisBio, Lilly, MedImmume, Medivation, Merck, Oncothyreon, Pfizer, Syndax, Tesaro; personal fees from Biotheranostics, Bristol Myers Squibb, Celgene, Daiichi Sankyo/Lilly, Eisai, Roche/Genentech, Novartis, NanoString Technologies. Dr. Untch has nothing to disclose. Dr. Huang reports grants to institution from Novartis, Daiichi Sankyo, AstraZeneca, EirGenix, Eli Lilly, MSD, OBI Pharma, Pfizer, F. Hoffmann-La Roche; personal fees from Novartis, Daiichi Sankyo, AstraZeneca, Eli Lilly, Pfizer, F. Hoffmann-La Roche; nonfinancial support from AstraZeneca, EirGenix, Eli Lilly, OBI Pharma, Roche, Novartis. Dr. Stroyakovskii has nothing to disclose. Dr. Xu reports personal fees from Novartis, AstraZeneca, Pfizer, Roche, Eisai. Dr. Moroose reports personal fees from Gilead, Lilly, Pfizer, Seagen, Genentech, Johnson & Johnson. Dr. Loi reports research funding grants to institution from Novartis, Bristol Myers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, AstraZeneca, Roche/Genentech, Seagen; uncompensated consultant for Seagen, Novartis, Bristol Myers Squibb, Merck, AstraZeneca, Eli Lilly, Pfizer, Gilead Therapeutics, Roche/Genentech; consultant with fees paid to institution from Aduro Biotech, Novartis, GSK, Roche/Genentech, AstraZeneca, Silverback Therapeutics, G1 Therapeutics, PUMA Biotechnologies, Pfizer, Gilead Therapeutics, Seagen, Daiichi Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly, Bristol Myers Squibb. Ms. Visco has nothing to disclose. Ms. Bee-Munteanu has nothing to disclose. Ms. Afenjar has nothing to disclose. Dr. Fresco reports other advising institution is contracted by Novartis as CRO conducting the NATALEE trial. Dr. Taran, Dr. Chakravartty, Dr. Zarate, Dr. Lteif report employment and stock ownership. Dr. Hortobagyi reports personal fees and grants to institution from Novartis.
Figures



References
-
- Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015; 313: 165–173. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Pistilli B, Lohrisch C, Sheade J, et al. Personalizing adjuvant endocrine therapy for early-stage hormone receptor-positive breast cancer. Am Soc Clin Oncol Educ Book. 2022; 42: 1–13. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

