A natural heme deficiency exists in biology that allows nitric oxide to control heme protein functions by regulating cellular heme distribution
- PMID: 37276366
- PMCID: PMC10478511
- DOI: 10.1002/bies.202300055
A natural heme deficiency exists in biology that allows nitric oxide to control heme protein functions by regulating cellular heme distribution
Abstract
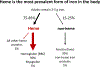
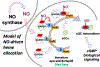
A natural heme deficiency that exists in cells outside of the circulation broadly compromises the heme contents and functions of heme proteins in cells and tissues. Recently, we found that the signaling molecule, nitric oxide (NO), can trigger or repress the deployment of intracellular heme in a concentration-dependent hormetic manner. This uncovers a new role for NO and sets the stage for it to shape numerous biological processes by controlling heme deployment and consequent heme protein functions in biology.
Keywords: anemia; evolution; heme; iron; nitric oxide; physiology.
© 2023 The Authors. BioEssays published by Wiley Periodicals LLC.
Figures
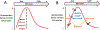
Comment in
-
Nitric oxide achieves master regulator status.Bioessays. 2023 Aug;45(8):e2300089. doi: 10.1002/bies.202300089. Epub 2023 Jun 15. Bioessays. 2023. PMID: 37318320 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

