CARD9 attenuates Aβ pathology and modifies microglial responses in an Alzheimer's disease mouse model
- PMID: 37276426
- PMCID: PMC10268238
- DOI: 10.1073/pnas.2303760120
CARD9 attenuates Aβ pathology and modifies microglial responses in an Alzheimer's disease mouse model
Abstract
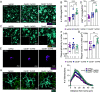
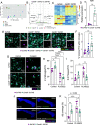
Recent advances have highlighted the importance of several innate immune receptors expressed by microglia in Alzheimer's disease (AD). In particular, mounting evidence from AD patients and experimental models indicates pivotal roles for TREM2, CD33, and CD22 in neurodegenerative disease progression. While there is growing interest in targeting these microglial receptors to treat AD, we still lack knowledge of the downstream signaling molecules used by these receptors to orchestrate immune responses in AD. Notably, TREM2, CD33, and CD22 have been described to influence signaling associated with the intracellular adaptor molecule CARD9 to mount downstream immune responses outside of the brain. However, the role of CARD9 in AD remains poorly understood. Here, we show that genetic ablation of CARD9 in the 5xFAD mouse model of AD results in exacerbated amyloid beta (Aβ) deposition, increased neuronal loss, worsened cognitive deficits, and alterations in microglial responses. We further show that pharmacological activation of CARD9 promotes improved clearance of Aβ deposits from the brains of 5xFAD mice. These results help to establish CARD9 as a key intracellular innate immune signaling molecule that regulates Aβ-mediated disease and microglial responses. Moreover, these findings suggest that targeting CARD9 might offer a strategy to improve Aβ clearance in AD.
Keywords: Alzheimer’s disease; CARD9; amyloid beta; microglia; neuroimmunology.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

