Pegaspargase-modified risk-oriented program for adult acute lymphoblastic leukemia: results of the GIMEMA LAL1913 trial
- PMID: 37276451
- PMCID: PMC10440455
- DOI: 10.1182/bloodadvances.2022009596
Pegaspargase-modified risk-oriented program for adult acute lymphoblastic leukemia: results of the GIMEMA LAL1913 trial
Abstract
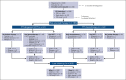
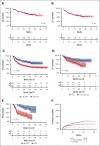
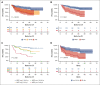
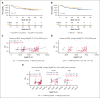
Pediatric-inspired chemotherapy is the standard of care for younger adults with Philadelphia chromosome-negative acute lymphoblastic leukemia/lymphoma (Ph- ALL/LL). In LAL1913 trial, the Gruppo Italiano Malattie EMatologiche dell'Adulto added pegaspargase 2000 IU/m2 to courses 1, 2, 5, and 6 of an 8-block protocol for patients aged from 18 to 65 years, with dose reductions in patients aged >55 years. Responders were risk stratified for allogeneic hematopoietic cell transplantation (HCT) or maintenance per clinical characteristics and minimal residual disease (MRD). Of 203 study patients (median age, 39.8 years), 91% achieved a complete remission. The 3-year overall survival, event-free, and disease-free survival (DFS) rates were 66.7%, 57.7%, and 63.3%, respectively, fulfilling the primary study end point of a 2-year DFS >55%. Although based on the intention-to-treat, the DFS being 74% and 50% in the chemotherapy (n = 94) and HCT (n = 91) assignment cohorts, respectively, a time-dependent analysis proved the value of HCT in patients who were eligible (DFS HCT 70% vs no HCT 26%; P <.0001). In multivariate analysis, age and MRD were independent factors predicting DFS rates of 86% (age ≤ 40 and MRD-negative), 64%-65% (MRD-positive or age > 40) and 25% (age > 40 and MRD-positive); P < .0001. Grade ≥2 pegaspargase toxicity was mainly observed at course 1, contributing to induction death in 2 patients but was rare thereafter. This program improved outcomes of patients with Ph- ALL/LL aged up to 65 years in a multicenter national setting. This trial was registered at www.clinicaltrials.gov as #NCT02067143.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.B. served on the speaker’s bureau and advisory board for Amgen, Incyte, Servier, Novartis, and Kite Pharma/ Gilead. S.C. served on advisory board for Amgen, Incyte, Kite Pharma/Gilead, and AbbVie. E.B. served as consultant for Janssen, as an advisory board member for AbbVie, and received travel grants from Incyte. G.M. received research support form Novartis, Bristol Myers Squibb, Amgen, Pfizer, Genzyme, and Celgene; served as consultant for Novartis, Bristol Myers Squibb, Amgen, Pfizer, Ariad Pharma, and Roche; served as speaker for Novartis, Bristol Myers Squibb, Amgen, Pfizer, Ariad Pharma, Genzyme, Celgene, Ariad, and Glaxo; and received honoraria from Novartis, Bristol Myers Squibb, Amgen, Pfizer, Genzyme, Celgene, and Ariad. N.F. served as advisory board member for, and received travel grants from, Amgen, Pfizer, AbbVie, and Incyte. M. Bocchia served as consultant and advisory board member for Incyte, Novartis, AstraZeneca, Janssen, and AbbVie. P.D.F. received fees as consultant from Jazz and Medac. M. Bonifacio received honoraria from BMS, Pfizer, and Incyte. A.C. received personal fees from AbbVie, Amgen, Gilead, Jazz, Pfizer, and Incyte. A.P. served as consultant for Takeda. A.R. served as consultant and on the speaker’s bureau for Amgen, Omeros, Novartis, Astellas, Jazz Pharmaceuticals, AbbVie, Janssen, Pfizer, Incyte, and Kite/Gilead. R.F. served on the speaker’s bureau for Amgen, Novartis, and AstraZeneca. The remaining authors declare no competing financial interests.
Figures


References
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. - PubMed
-
- Bassan R, Bourquin JP, DeAngelo DJ, Chiaretti S. New approaches to the management of adult acute lymphoblastic leukemia. J Clin Oncol. 2018;36(35):3504–3519. - PubMed
-
- Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. - PubMed
-
- Huguet F, Chevret S, Leguay T, et al. Group of Research on Adult ALL (GRAALL) Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36(24):2514–2523. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

