Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study
- PMID: 37277275
- PMCID: PMC10527050
- DOI: 10.1016/j.eururo.2023.05.021
Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study
Abstract
Background: Initial TRITON2 (NCT02952534) results demonstrated the efficacy of rucaparib 600 mg BID in patients with metastatic castration-resistant prostate cancer (mCRPC) associated with a BRCA1 or BRCA2 (BRCA) or other DNA damage repair (DDR) gene alteration.
Objective: To present the final data from TRITON2.
Design, setting, and participants: TRITON2 enrolled patients with mCRPC who had progressed on one or two lines of next-generation androgen receptor-directed therapy and one taxane-based chemotherapy.
Outcome measurements and statistical analysis: The primary endpoint was objective response rate (ORR; as per the modified Response Evaluation Criteria in Solid Tumor Version 1.1/Prostate Cancer Clinical Trials Working Group 3 criteria in patients with measurable disease by independent radiology review [IRR]); prostate-specific antigen (PSA) response rate (≥50% decrease from baseline [PSA50]) was a key secondary endpoint.
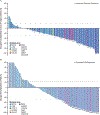
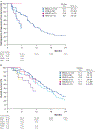
Results and limitations: As of July 27, 2021 (study closure), TRITON2 had enrolled 277 patients, grouped by mutated gene: BRCA (n = 172), ATM (n = 59), CDK12 (n = 15), CHEK2 (n = 7), PALB2 (n = 11), or other DDR gene (Other; n = 13). ORR by IRR was 46% (37/81) in the BRCA subgroup (95% confidence interval [CI], 35-57%), 100% (4/4) in the PALB2 subgroup (95% CI, 40-100%), and 25% (3/12) in the Other subgroup (95% CI, 5.5-57%). No patients within the ATM, CDK12, or CHEK2 subgroups had an objective response by IRR. PSA50 response rates (95% CI) in the BRCA, PALB2, ATM, CDK12, CHEK2, and Other subgroups were 53% (46-61%), 55% (23-83%), 3.4% (0.4-12), 6.7% (0.2-32%), 14% (0.4-58%), and 23% (5.0-54%), respectively.
Conclusions: The final TRITON2 results confirm the clinical benefit and manageable safety profile of rucaparib in patients with mCRPC, including those with an alteration in BRCA or select non-BRCA DDR gene.
Patient summary: Almost half of TRITON2 patients with BRCA-mutated metastatic castration-resistant prostate cancer had a complete or partial tumor size reduction with rucaparib; clinical benefits were also observed with other DNA damage repair gene alterations.
Keywords: DNA damage repair gene alteration; Metastatic castration-resistant prostate cancer; Poly(ADP-ribose) polymerase inhibitor.
Crown Copyright © 2023. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Rucaparib monotherapy in the heavily pre-treated metastatic castrate-resistant prostate cancer setting: practical considerations and alternate treatment approaches.Transl Androl Urol. 2024 May 31;13(5):884-888. doi: 10.21037/tau-23-671. Epub 2024 May 17. Transl Androl Urol. 2024. PMID: 38855585 Free PMC article. No abstract available.
References
-
- Merseburger AS, Waldron N, Ribal MJ, et al. Genomic testing in patients with metastatic castration-resistant prostate cancer: a pragmatic guide for clinicians. Eur Urol 2021;79:519–29. - PubMed
-
- Rubraca® (rucaparib) tablets [prescribing information]. Boulder, CO: Clovis Oncology, Inc.; 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

