Monitoring transmission intensity of trachoma with serology
- PMID: 37277341
- PMCID: PMC10241377
- DOI: 10.1038/s41467-023-38940-5
Monitoring transmission intensity of trachoma with serology
Abstract
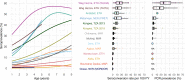
Trachoma, caused by ocular Chlamydia trachomatis infection, is targeted for global elimination as a public health problem by 2030. To provide evidence for use of antibodies to monitor C. trachomatis transmission, we collated IgG responses to Pgp3 antigen, PCR positivity, and clinical observations from 19,811 children aged 1-9 years in 14 populations. We demonstrate that age-seroprevalence curves consistently shift along a gradient of transmission intensity: rising steeply in populations with high levels of infection and active trachoma and becoming flat in populations near elimination. Seroprevalence (range: 0-54%) and seroconversion rates (range: 0-15 per 100 person-years) correlate with PCR prevalence (r: 0.87, 95% CI: 0.57, 0.97). A seroprevalence threshold of 13.5% (seroconversion rate 2.75 per 100 person-years) identifies clusters with any PCR-identified infection at high sensitivity ( >90%) and moderate specificity (69-75%). Antibody responses in young children provide a robust, generalizable approach to monitor population progress toward and beyond trachoma elimination.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Monitoring transmission intensity of trachoma with serology.medRxiv [Preprint]. 2023 May 17:2023.02.13.23285881. doi: 10.1101/2023.02.13.23285881. medRxiv. 2023. Update in: Nat Commun. 2023 Jun 5;14(1):3269. doi: 10.1038/s41467-023-38940-5. PMID: 36824972 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. Ending the neglect to attain the sustainable development goals: a roadmap for neglected tropical diseases 2021–2030. https://apps.who.int/iris/handle/10665/361856 (2020).

