Parallel interrogation of the chalcogenide-based micro-ring sensor array for photoacoustic tomography
- PMID: 37277353
- PMCID: PMC10241812
- DOI: 10.1038/s41467-023-39075-3
Parallel interrogation of the chalcogenide-based micro-ring sensor array for photoacoustic tomography
Abstract
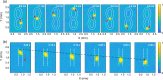
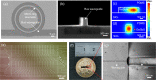
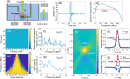
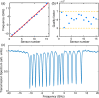
Photoacoustic tomography (PAT), also known as optoacoustic tomography, is an attractive imaging modality that provides optical contrast with acoustic resolutions. Recent progress in the applications of PAT largely relies on the development and employment of ultrasound sensor arrays with many elements. Although on-chip optical ultrasound sensors have been demonstrated with high sensitivity, large bandwidth, and small size, PAT with on-chip optical ultrasound sensor arrays is rarely reported. In this work, we demonstrate PAT with a chalcogenide-based micro-ring sensor array containing 15 elements, while each element supports a bandwidth of 175 MHz (-6 dB) and a noise-equivalent pressure of 2.2 mPaHz-1/2. Moreover, by synthesizing a digital optical frequency comb (DOFC), we further develop an effective means of parallel interrogation to this sensor array. As a proof of concept, parallel interrogation with only one light source and one photoreceiver is demonstrated for PAT with this sensor array, providing images of fast-moving objects, leaf veins, and live zebrafish. The superior performance of the chalcogenide-based micro-ring sensor array and the effectiveness of the DOFC-enabled parallel interrogation offer great prospects for advancing applications in PAT.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Taruttis A, Ntziachristos V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat. Photonics. 2015;9:219–227. doi: 10.1038/nphoton.2015.29. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

