Sexual differentiation in human malaria parasites is regulated by competition between phospholipid metabolism and histone methylation
- PMID: 37277533
- PMCID: PMC11163918
- DOI: 10.1038/s41564-023-01396-w
Sexual differentiation in human malaria parasites is regulated by competition between phospholipid metabolism and histone methylation
Abstract
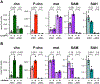
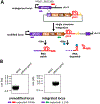
For Plasmodium falciparum, the most widespread and virulent malaria parasite that infects humans, persistence depends on continuous asexual replication in red blood cells, while transmission to their mosquito vector requires asexual blood-stage parasites to differentiate into non-replicating gametocytes. This decision is controlled by stochastic derepression of a heterochromatin-silenced locus encoding AP2-G, the master transcription factor of sexual differentiation. The frequency of ap2-g derepression was shown to be responsive to extracellular phospholipid precursors but the mechanism linking these metabolites to epigenetic regulation of ap2-g was unknown. Through a combination of molecular genetics, metabolomics and chromatin profiling, we show that this response is mediated by metabolic competition for the methyl donor S-adenosylmethionine between histone methyltransferases and phosphoethanolamine methyltransferase, a critical enzyme in the parasite's pathway for de novo phosphatidylcholine synthesis. When phosphatidylcholine precursors are scarce, increased consumption of SAM for de novo phosphatidylcholine synthesis impairs maintenance of the histone methylation responsible for silencing ap2-g, increasing the frequency of derepression and sexual differentiation. This provides a key mechanistic link that explains how LysoPC and choline availability can alter the chromatin status of the ap2-g locus controlling sexual differentiation.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures

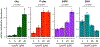
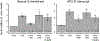










Similar articles
-
Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites.Nature. 2017 Nov 2;551(7678):95-99. doi: 10.1038/nature24280. Epub 2017 Sep 25. Nature. 2017. PMID: 29094698 Free PMC article.
-
Plasmodium falciparum gametocytes display global chromatin remodelling during sexual differentiation.BMC Biol. 2023 Apr 3;21(1):65. doi: 10.1186/s12915-023-01568-4. BMC Biol. 2023. PMID: 37013531 Free PMC article.
-
A transcriptional switch underlies commitment to sexual development in malaria parasites.Nature. 2014 Mar 13;507(7491):248-52. doi: 10.1038/nature12920. Epub 2014 Feb 23. Nature. 2014. PMID: 24572369 Free PMC article.
-
Metabolic regulation of sexual commitment in Plasmodium falciparum.Curr Opin Microbiol. 2020 Dec;58:93-98. doi: 10.1016/j.mib.2020.09.004. Epub 2020 Oct 11. Curr Opin Microbiol. 2020. PMID: 33053503 Free PMC article. Review.
-
Histone globular domain epigenetic modifications: The regulators of chromatin dynamics in malaria parasite.Chembiochem. 2024 Feb 16;25(4):e202300596. doi: 10.1002/cbic.202300596. Epub 2024 Jan 19. Chembiochem. 2024. PMID: 38078518 Review.
Cited by
-
Genome-wide profiling of histone modifications in Plasmodium falciparum using CUT&RUN.Life Sci Alliance. 2022 Nov 15;6(1):e202201778. doi: 10.26508/lsa.202201778. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36379668 Free PMC article.
-
PfEMP1 and var genes - Still of key importance in Plasmodium falciparum malaria pathogenesis and immunity.Adv Parasitol. 2024;125:53-103. doi: 10.1016/bs.apar.2024.02.001. Epub 2024 Mar 23. Adv Parasitol. 2024. PMID: 39095112 Free PMC article. Review.
-
Phenotypic Screens Identify Genetic Factors Associated with Gametocyte Development in the Human Malaria Parasite Plasmodium falciparum.Microbiol Spectr. 2023 Jun 15;11(3):e0416422. doi: 10.1128/spectrum.04164-22. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154686 Free PMC article.
-
Hungry for control: metabolite signaling to chromatin in Plasmodium falciparum.Curr Opin Microbiol. 2024 Apr;78:102430. doi: 10.1016/j.mib.2024.102430. Epub 2024 Feb 2. Curr Opin Microbiol. 2024. PMID: 38306915 Free PMC article. Review.
-
Plasmodium falciparum Sexual Commitment Rate Variation among Clinical Isolates and Diverse Laboratory-Adapted Lines.Microbiol Spectr. 2022 Dec 21;10(6):e0223422. doi: 10.1128/spectrum.02234-22. Epub 2022 Nov 21. Microbiol Spectr. 2022. PMID: 36409095 Free PMC article.
References
-
- Drakeley C, Sutherland C, Bousema JT, Sauerwein RW & Targett GAT The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends in Parasitology 22, 424–430 (2006). - PubMed
-
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, Kafsack BFC, Williams AE, Llinás M, Berriman M, Billker O & Waters AP A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 507, 253–257 (2014). - PMC - PubMed
-
- Brancucci NMB, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z & Voss TS Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 16, 165–176 (2014). - PubMed
-
- Fraschka SA, Filarsky M, Hoo R, Niederwieser I, Yam XY, Brancucci NMB, Mohring F, Mushunje AT, Huang X, Christensen PR, Nosten F, Bozdech Z, Russell B, Moon RW, Marti M, Preiser PR, Bartfai R & Voss TS Comparative Heterochromatin Profiling Reveals Conserved and Unique Epigenome Signatures Linked to Adaptation and Development of Malaria Parasites. Cell Host Microbe 23, 407–420.e8 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

