Paediatric inflammatory multisystem syndrome in Canada: population-based surveillance and role of SARS-CoV-2 linkage
- PMID: 37277605
- PMCID: PMC10241135
- DOI: 10.1038/s41390-023-02668-1
Paediatric inflammatory multisystem syndrome in Canada: population-based surveillance and role of SARS-CoV-2 linkage
Abstract
Background: Paediatric inflammatory multisystem syndrome (PIMS) is a rare condition temporally associated with SARS-CoV-2 infection. Using national surveillance data, we compare presenting features and outcomes among children hospitalized with PIMS by SARS-CoV-2 linkage, and identify risk factors for intensive care (ICU).
Methods: Cases were reported to the Canadian Paediatric Surveillance Program by a network of >2800 pediatricians between March 2020 and May 2021. Patients with positive versus negative SARS-CoV-2 linkages were compared, with positive linkage defined as any positive molecular or serologic test or close contact with confirmed COVID-19. ICU risk factors were identified with multivariable modified Poisson regression.
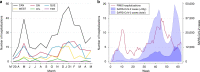
Results: We identified 406 children hospitalized with PIMS, including 49.8% with positive SARS-CoV-2 linkages, 26.1% with negative linkages, and 24.1% with unknown linkages. The median age was 5.4 years (IQR 2.5-9.8), 60% were male, and 83% had no comorbidities. Compared to cases with negative linkages, children with positive linkages experienced more cardiac involvement (58.8% vs. 37.4%; p < 0.001), gastrointestinal symptoms (88.6% vs. 63.2%; p < 0.001), and shock (60.9% vs. 16.0%; p < 0.001). Children aged ≥6 years and those with positive linkages were more likely to require ICU.
Conclusions: Although rare, 30% of PIMS hospitalizations required ICU or respiratory/hemodynamic support, particularly those with positive SARS-CoV-2 linkages.
Impact: We describe 406 children hospitalized with paediatric inflammatory multisystem syndrome (PIMS) using nationwide surveillance data, the largest study of PIMS in Canada to date. Our surveillance case definition of PIMS did not require a history of SARS-CoV-2 exposure, and we therefore describe associations of SARS-CoV-2 linkages on clinical features and outcomes of children with PIMS. Children with positive SARS-CoV-2 linkages were older, had more gastrointestinal and cardiac involvement, and hyperinflammatory laboratory picture. Although PIMS is rare, one-third required admission to intensive care, with the greatest risk amongst those aged ≥6 years and those with a SARS-CoV-2 linkage.
© 2023. The Author(s), under exclusive licence to the International Pediatric Research Foundation, Inc.
Conflict of interest statement
M.-P.M. has received consulting fees from Sobin and AbbVie and payment for expert testimony from the Canadian Medical Protective Association. S.K.M. has received honoraria for lectures from GlaxoSmithKline, was a member of ad hoc advisory boards for Pfizer Canada and Sanofi Pasteur, and is an investigator on an investigator-led grant from Pfizer. R.A.B. has received honoraria and participated in advisory boards with SOBI, Roche, Amgen, and AbbVie. K.B. served as Past President of the Community Paediatrics Section of the Canadian Paediatric Society and has received royalties from Brush Education. K.C. is Chair of the Acute Care Committee of the Canadian Paediatric Society and is past president of the Emergency Medicine Section of the Canadian Paediatric Society. E.J.D. is Chair of the Scientific Research Committee and a director of Epilepsy Canada. She is also a member of Partners Against Mortality in Epilepsy and the advisory boards of Cardiol, Pendopharm and Stoke Therapeutics. C.F. is Chair of the Scientific Steering Committee for the Canadian Paediatric Surveillance Program, former Chair of the Specialty Committee in Pediatrics of the Royal College of Physicians and Surgeons of Canada, former President of the Canadian Paediatric Society, and member of the Executive as Secretary of the Canadian Critical Care Society. She has received reimbursement for travel expenses from Canadian Paediatric Society and the Royal College of Physicians and Surgeons of Canada. She has also received an honorarium for a presentation at a continuing education conference from the Université de Sherbrooke. S.F. is the President of the Association of Medical Microbiology and Infectious Disease Canada and has received consulting fees from Toronto Metropolitan University. F.K. has received honoraria for presentations given to the Association des Pédiatres du Québec and receives CMV testing kits from Altona Diagnostics. R.M.L. has received honoraria for serving as a consultant to Sobi, Novartis, Sanofi, and Eli Lilly, as chair for data monitoring committees for Eli Lilly and Novartis, and from the Canadian Rheumatology Association. C.M.H. is the Director of Children’s Mental Health of Ontario, and the Director of medical affairs for the Canadian Paediatric Society and the Canadian Paediatric Surveillance Program. J.P. reports grants from MedImmune, grants and personal fees from Merck and AbbVie, and personal fees from AstraZeneca, all outside the submitted work. R.P. is a consultant for Verity Pharmaceuticals. M.S. is supported via salary awards from the BC Children’s Hospital Foundation and the Michael Smith Foundation for Health Researc and has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. M.L.S. is an employee of the Public Health Agency of Canada. R.S. has received honoraria and served on an advisory board and as a consultant with Novartis, honoraria from Canadian Rheumatology Association, is a board member for Rheumatology for All, and her institution receives funding from Bristol Myers Squibb for a patient registry for which she is PO. E.H. has participated in advisory board meetings of CSL-Behring and Takeda, data safety monitoring boards of Rocket Pharmaceutical and Jasper Therapeutics, and has a patent application with the biotech Immugenia and the biotech Immune Biosolutions. All other authors report no declaration of interests.
Figures

References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

