The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets
- PMID: 37277609
- PMCID: PMC10290954
- DOI: 10.1038/s42255-023-00811-0
The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets
Abstract
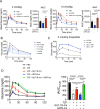
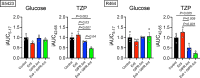
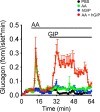
The incretins glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) mediate insulin responses that are proportionate to nutrient intake to facilitate glucose tolerance1. The GLP-1 receptor (GLP-1R) is an established drug target for the treatment of diabetes and obesity2, whereas the therapeutic potential of the GIP receptor (GIPR) is a subject of debate. Tirzepatide is an agonist at both the GIPR and GLP-1R and is a highly effective treatment for type 2 diabetes and obesity3,4. However, although tirzepatide activates GIPR in cell lines and mouse models, it is not clear whether or how dual agonism contributes to its therapeutic benefit. Islet beta cells express both the GLP-1R and the GIPR, and insulin secretion is an established mechanism by which incretin agonists improve glycemic control5. Here, we show that in mouse islets, tirzepatide stimulates insulin secretion predominantly through the GLP-1R, owing to reduced potency at the mouse GIPR. However, in human islets, antagonizing GIPR activity consistently decreases the insulin response to tirzepatide. Moreover, tirzepatide enhances glucagon secretion and somatostatin secretion in human islets. These data demonstrate that tirzepatide stimulates islet hormone secretion from human islets through both incretin receptors.
© 2023. The Author(s).
Conflict of interest statement
The Campbell group receives funding for basic science from Novo Nordisk and Eli Lilly. The Müller group receives funding for basic science from Novo Nordisk. F.S.W., D.B.W. and K.W.S. are employees of Eli Lilly. J.D.D., B.Y. and B.F. are employees of Novo Nordisk. D.A.D. has served as a consultant or speaker within the past 12 months for Eli Lilly and Structure Therapeutics. J.E.C. has served as a consultant or speaker within the past 12 months for Structure Therapeutics. The remaining authors declare no competing interests or conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

