The role of peripheral inflammatory insults in Alzheimer's disease: a review and research roadmap
- PMID: 37277738
- PMCID: PMC10240487
- DOI: 10.1186/s13024-023-00627-2
The role of peripheral inflammatory insults in Alzheimer's disease: a review and research roadmap
Abstract
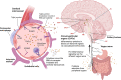
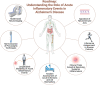
Peripheral inflammation, defined as inflammation that occurs outside the central nervous system, is an age-related phenomenon that has been identified as a risk factor for Alzheimer's disease. While the role of chronic peripheral inflammation has been well characterized in the context of dementia and other age-related conditions, less is known about the neurologic contribution of acute inflammatory insults that take place outside the central nervous system. Herein, we define acute inflammatory insults as an immune challenge in the form of pathogen exposure (e.g., viral infection) or tissue damage (e.g., surgery) that causes a large, yet time-limited, inflammatory response. We provide an overview of the clinical and translational research that has examined the connection between acute inflammatory insults and Alzheimer's disease, focusing on three categories of peripheral inflammatory insults that have received considerable attention in recent years: acute infection, critical illness, and surgery. Additionally, we review immune and neurobiological mechanisms which facilitate the neural response to acute inflammation and discuss the potential role of the blood-brain barrier and other components of the neuro-immune axis in Alzheimer's disease. After highlighting the knowledge gaps in this area of research, we propose a roadmap to address methodological challenges, suboptimal study design, and paucity of transdisciplinary research efforts that have thus far limited our understanding of how pathogen- and damage-mediated inflammatory insults may contribute to Alzheimer's disease. Finally, we discuss how therapeutic approaches designed to promote the resolution of inflammation may be used following acute inflammatory insults to preserve brain health and limit progression of neurodegenerative pathology.
Keywords: Cytokines; Dementia; Infection; Inflammation; Neuro-immune axis; Neuroinflammation; Peripheral inflammation; Systemic inflammation.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Prince, M. et al. World Alzheimer Report 2015: The global impact of dementia - an analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int 2015;84.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

