Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer
- PMID: 37277863
- PMCID: PMC10242978
- DOI: 10.1186/s13046-023-02720-2
Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer
Abstract
Introduction: Cuproptosis and ferroptosis are the two newly defined metal-related regulated cell death. However, the crosstalk between cuproptosis and ferroptosis is obscure.
Materials and methods: We analyzed the effect of ferroptosis inducers on copper ionophores-induced cell death through CCK-8 assay. Cuproptosis was studied using immunofluorescence and protein soluble-insoluble fraction isolation. GSH assay, qRT-PCR and western blot were adopted to explore the machinery of ferroptosis inducers enhanced cuproptosis. And mouse xenograft model was built to detect the synergy effect of elesclomol-Cu and sorafenib in vivo.
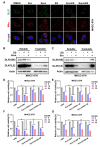
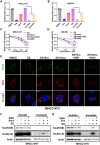
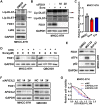
Results: Herein we found that ferroptosis inducers sorafenib and erastin could enhance cuproptosis in primary liver cancer cells by increasing copper dependent lipoylated protein aggregation. Mechanically, sorafenib and erastin upregulated protein lipoylation via suppressing mitochondrial matrix-related proteases mediated ferredoxin 1 (FDX1) protein degradation, and reduced intracellular copper chelator glutathione (GSH) synthesis through inhibiting cystine importing.
Discussion/conclusion: Our findings proposed that combination of ferroptosis inducers and copper ionophores to co-targeting ferroptosis and cuproptosis could be a novel therapeutic strategy for primary liver cancer.
Keywords: Copper ionophores; Cuproptosis; Ferredoxin 1 (FDX1); Ferroptosis; Lipoylation.
© 2023. The Author(s).
Conflict of interest statement
There is no conflict of interest to declare.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

