A Complex Intervention to Prevent Medication-Related Hospital Admissions
- PMID: 37278031
- PMCID: PMC10478767
- DOI: 10.3238/arztebl.m2023.0123
A Complex Intervention to Prevent Medication-Related Hospital Admissions
Abstract
Background: Children are often treated off-label and are at a disadvantage in pharmacotherapy. The aim of this study was to implement and evaluate a quality assurance measure (PaedPharm) for pediatric pharmacotherapy whose purpose is to reduce medication-related hospitalizations among children and adolescents.
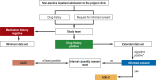
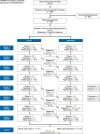
Methods: PaedPharm consisted of the digital pediatric drug information system PaedAMIS, pediatric pharmaceutical quality circles (PaedZirk), and an adverse drug event (ADE) reporting system (PaedReport). The intervention was implemented in a cluster-randomized trial (DRKS 00013924) in 12 regions, with a pediatric and adolescent medicine clinic in each and a total of 152 surrounding private practitioners, in 6 sequences over 8 quarters. In addition to the proportion of ADE-related hospital admissions (primary endpoint), comprehensive process evaluation included other endpoints such as coverage, user acceptance, and relevance to practice.
Results: 41 829 inpatient admissions were recorded, of which 5101 were patients of physicians who participated in our study. 4.1% of admissions were ADE-related under control conditions, and 3.1% under intervention conditions (95% CI: [2.3; 5.9] and [1.8; 4.5], respectively). A model-based comparison yielded an intervention effect of 0.73 (population-based odds ratio; [0.39; 1.37]; p = 0.33). PaedAMIS achieved moderate user acceptance and PaedZirk achieved high user acceptance.
Conclusion: The introduction of PaedPharm was associated with a decrease in medication-related hospitalizations that did not reach statistical significance. The process evaluation revealed broad acceptance of the intervention in outpatient pediatrics and adolescent medicine.
Figures




Comment in
-
Pharmacotherapy for Children and Adolescents.Dtsch Arztebl Int. 2023 Jun 23;120(25):423-424. doi: 10.3238/arztebl.m2023.0146. Dtsch Arztebl Int. 2023. PMID: 37661332 Free PMC article. No abstract available.
References
-
- Kimland E, Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91:796–801. - PubMed
-
- Magalhaes J, Rodrigues AT, Roque F, Figueiras A, Falcao A, Herdeiro MT. Use of off-label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol. 2015;71:1–13. - PubMed
-
- Andrade SRA, Santos P, Andrade PHS, da Silva WB, Unlicensed and off-label prescription of drugs to children in primary health care: a systematic review J Evid Based Med. 2020;13:292–300. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

