Questionnaire PLD-complaint-specific assessment identifies need for therapy in polycystic liver disease: A multi-centric prospective study
- PMID: 37278135
- PMCID: PMC10493353
- DOI: 10.1002/ueg2.12387
Questionnaire PLD-complaint-specific assessment identifies need for therapy in polycystic liver disease: A multi-centric prospective study
Abstract
Background and aims: Polycystic liver disease (PLD) can lead to extensive hepatomegaly. Symptom relief is the primary goal of the treatment. The role of the recently developed disease-specific questionnaires for identification of the thresholds and the assessment of therapy needs further investigation.
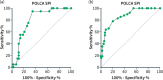
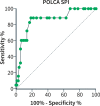
Methods: A five-year prospective multi-centric observational study in 21 hospitals in Belgium gathered a study population of 198 symptomatic PLD-patients of whom the disease-specific symptom questionnaire PLD-complaint-specific assessment (POLCA) scores were calculated. The thresholds of the POLCA score for the need for volume reduction therapy were analyzed.
Results: The study group consisted of mostly (82.8%) women with baseline mean age of 54.4 years ±11.2, median liver volume expressed as height-adjusted total liver volume(htLV) of 1994 mL (interquartile range [IQR] 1275; 3150) and median growth of the liver of +74 mL/year (IQR +3; +230). Volume reduction therapy was needed in 71 patients (35.9%). A POLCA severity score (SPI) ≥ 14 predicted the need for therapy both in the derivation (n = 63) and the validation cohort (n = 126). The thresholds to start somatostatin analogues (n = 55) or to consider liver transplantation (n = 18) were SPI scores of ≥14 and ≥ 18 and the corresponding mean htLVs were 2902 mL (IQR 1908; 3964) and 3607 mL (IQR 2901; 4337), respectively. Somatostatin analogues treatment resulted in a decrease in the SPI score -6.0 versus + 4.5 in patients without somatostatin analogues (p < 0.01). Changes in the SPI score were significantly different between the liver transplantation group and no liver transplantation group, +4.3 ± 7.1 versus -1.6 ± 4.9, respectively, (p < 0.01).
Conclusion: A polycystic liver disease-specific questionnaire can be used as a guide on when to start a volume reduction therapy and to assess the effect of treatment.
Keywords: health-related quality of life; liver transplantation; liver volume; polycystic liver disease; somatostatin analogues.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Frederik Nevens: Research grant from Ipsen; all other authors declare no competing interests.
Figures
Comment in
-
Patient reported outcome measure thresholds: The next step in the management of polycystic liver disease.United European Gastroenterol J. 2023 Jun;11(5):403-404. doi: 10.1002/ueg2.12394. Epub 2023 May 1. United European Gastroenterol J. 2023. PMID: 37128131 Free PMC article. No abstract available.
References
-
- EASL clinical practice guidelines on management of cystic liver diseases. J Hepatol 2022. S0168‐8278(22)00347‐6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

