Predictors of 6-year event-free survival in Alagille syndrome patients treated with maralixibat, an ileal bile acid transporter inhibitor
- PMID: 37278241
- PMCID: PMC10653287
- DOI: 10.1097/HEP.0000000000000502
Predictors of 6-year event-free survival in Alagille syndrome patients treated with maralixibat, an ileal bile acid transporter inhibitor
Abstract
Background and aims: Refractory pruritus and other complications of cholestasis are indications for liver transplantation (LT) in patients with Alagille syndrome (ALGS). We evaluated predictors of event-free survival and transplant-free survival in patients with ALGS treated with maralixibat (MRX), an ileal bile acid transporter inhibitor.
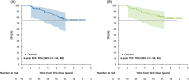
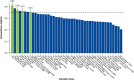
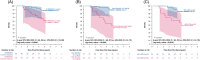
Approach and results: We assessed patients with ALGS from 3 clinical trials of MRX with up to 6 years of follow-up. Event-free survival was defined as the absence of LT, surgical biliary diversion, hepatic decompensation, or death; transplant-free survival was the absence of LT or death. Forty-three potential predictors were evaluated, including age, pruritus (ItchRO[Obs] 0-4 scale), biochemistries, platelets, and serum bile acids. Harrell's concordance statistic assessed goodness-of-fit, and then, Cox proportional hazard models confirmed the statistical significance of the predictors identified. A further analysis was performed to identify cutoffs using a grid search. Seventy-six individuals met the criteria of receiving MRX for ≥48 weeks with laboratory values available at week 48 (W48). The median duration of MRX was 4.7 years (IQR: 1.6-5.8); 16 had events (10 LT, 3 decompensation, 2 death, and 1 surgical biliary diversion). The 6-year event-free survival improved with a clinically meaningful >1-point ItchRO(Obs) reduction from baseline to W48 (88% vs. 57%; p = 0.005), W48 bilirubin < 6.5 mg/dL (90% vs. 43%; p < 0.0001), and W48 serum bile acid < 200 µmol/L (85% vs. 49%; p = 0.001). These parameters were also predictive of 6-year transplant-free survival.
Conclusions: Improvement in pruritus by 48 weeks, and lower W48 bilirubin and serum bile acid levels were associated with fewer events. These data may help identify potential markers of disease progression for ALGS patients treated with MRX.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Ronald J. Sokol advises and consults for Albireo. He advises Mirum. He consults for Alexion. Emmanuel M. Gonzales consults for and advises Albireo and Mirum. He consults for Laboratoires CTRS and Vivet Therapeutics. Binita M. Kamath consults for and received grants from Mirum and Albireo. She consults for Audentes. Alastair Baker received grants from Mirum and Albireo. Pamela Vig owns stock in and is employed by Mirum. Douglas B. Mogul owns stock in and is employed by Mirum. Will Garner owns stock in and is employed by Mirum. Bettina E. Hansen consults for and received grants from Intercept, Calliditas, and Cymabay. She consults for Ipsen, Alberio, Mirum, Enyo, and Eiger. Emmanuel Jacquemin consults for Laboratoire CTRS and Vivet Therapeutics. Richard J. Thompson consults for and owns stock in GenerationBio and Rectify Therapeutics. He consults for Mirum and Albireo.
Figures

References
-
- Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

