Liraglutide ameliorates oxidized LDL-induced endothelial dysfunction by GLP-1R-dependent downregulation of LOX-1-mediated oxidative stress and inflammation
- PMID: 37278349
- PMCID: PMC10249460
- DOI: 10.1080/13510002.2023.2218684
Liraglutide ameliorates oxidized LDL-induced endothelial dysfunction by GLP-1R-dependent downregulation of LOX-1-mediated oxidative stress and inflammation
Abstract
Objective: To investigate the effects of glucagon-like peptide 1 receptor (GLP-1R) agonist liraglutide on endothelial dysfunction in LDL receptor-deficient (LDLR-KO) mice and ox-LDL-challenged human umbilical vein endothelial cells (HUVECs) and its possible mechanism.
Methods: LDLR-KO mice were randomly treated with normal saline, liraglutide, or liraglutide plus a GLP-1R antagonist exendin-9 for four weeks. In parallel, HUVECs were cultured with ox-LDL alone or combined with liraglutide, in the presence or absence of lectin-like ox-LDL receptor-1(LOX-1) overexpression or GLP-1R knockdown. Endothelial-dependent relaxation and LOX-1 protein expression of thoracic aorta, circulating levels of oxidative and inflammatory markers in mice, and cell survival, reactive oxygen species production, and expression of adhesion molecules and signal regulators in ox-LDL cultured endothelial cells were measured.
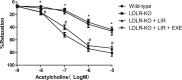
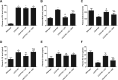
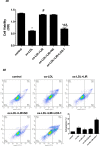
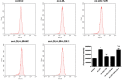
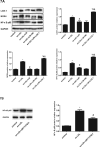
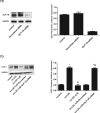
Results: liraglutide effectively enhanced acetylcholine-induced vasodilation, reduced LOX-1 expression in aortas, and decreased circulatory oxidative and inflammatory levels in LDLR-KO mice, which were abolished by cotreatment with exendin-9. HUVECs exposed to ox-LDL exhibited reduced cell viability, increased reactive oxygen species production and apoptosis, and elevated protein expression of ICAM-1, VCAM-1, LOX-1, NOX4, and NF-κB, which were markedly ameliorated by liraglutide treatment. The protective effects of liraglutide against ox-LDL-induced cell injury were abrogated in HUVECs overexpressing LOX-1 or silencing GLP-1R.
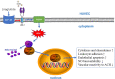
Conclusions: Liraglutide improved oxidized LDL-induced endothelial dysfunction via GLP-1R-dependent downregulation of LOX-1-mediated oxidative stress and inflammation.
Keywords: Liraglutide; endothelial dysfunction; inflammation; lectin-like ox-LDL receptor-1; oxidative stress; oxidized low-density lipoprotein.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Xu S, Ilyas I, Little PJ, et al. . Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 2021;73(3):924–967. - PubMed
-
- Murdaca G, Colombo BM, Cagnati P, et al. . Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224(2):309–317. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
