Palmerston North Interventional Rapid Avastin Treat and Extend (PIRATE) study protocol; meeting the medical retina service needs of our ageing population
- PMID: 37278418
- PMCID: PMC9872453
- DOI: 10.1136/bmjophth-2022-001192
Palmerston North Interventional Rapid Avastin Treat and Extend (PIRATE) study protocol; meeting the medical retina service needs of our ageing population
Abstract
Introduction: As the rates of age-related macular denegation exponentially increase, new innovation is required to address the challenges faced by our ageing population. The aim of the Palmerston North Interventional Rapid Avastin Treat and Extend (PIRATE) study is to establish the safety and efficacy of rapid treatment extension of bevacizumab (Avastin) in patients with low-risk neovascular age-related macular degeneration (nAMD).
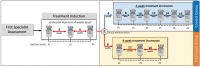
Methods and analysis: The PIRATE study is a monocentric, non-blinded, open-label randomised control trial. Participants over the age of 50 years with low-risk nAMD characteristics will be recruited in a prospective manner and randomised into treatment and control groups. Rapid treatment extension by 4 weeks will be applied in the treatment group, with the standard 2-week treatment extension occurring among controls. Participants will enter the trial after initial treatment induction consisting of three bevacizumab injections, 1 month apart. The primary outcome of best-corrected visual acuity will be assessed along with predetermined secondary outcomes at a study duration of 12 months (initial) and 24 months (total).
Trial registration number: ACTRN12622001246774p.
Keywords: Clinical Trial; Macula; Retina; Vision.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- National Health Committee . Age-Related macular degeneration. Wellington, New Zealand: National health Committee, 2015. Available: http://nhc.health.govt.nz [Accessed 8 Oct 2022].
-
- Macular Degeneration New Zealand . Socioeconomic cost of macular degeneration in New Zealand. Deloitte access economics. Available: https://static1.squarespace.com/static/5dcba244e3fb8952b16c79fd/t/6119d7... [Accessed 8 Oct 2022].
-
- RANZCO . NZ national guidelines for management for neovascular. New Zealand: AMD, 2018. https://ranzco.edu/policies_and_guideli/nz-national-guidelines-for-manag...
-
- Ohji M, Takahashi K, Okada AA, et al. . Efficacy and Safety of Intravitreal Aflibercept Treat-and-Extend Regimens in Exudative Age-Related Macular Degeneration: 52- and 96-Week Findings from ALTAIR : A Randomized Controlled Trial. Adv Ther 2020;37:1173–87. 10.1007/s12325-020-01236-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials