Sociodemographic disparities in ophthalmological clinical trials
- PMID: 37278426
- PMCID: PMC9950885
- DOI: 10.1136/bmjophth-2022-001175
Sociodemographic disparities in ophthalmological clinical trials
Abstract
Introduction: In ophthalmology, clinical trials (CTs) guide the treatment of diseases such as diabetic retinopathy, myopia, age-related macular degeneration, glaucoma and keratoconus with distinct presentations, pathological characteristics and responses to treatment in minority populations.Reporting gender and race and ethnicity in healthcare studies is currently recommended by National Institutes of Health (NIH) and Food and Drug Administration (FDA) guidelines to ensure representativeness and generalisability; however, CT results that include this information have been limited in the past 30 years.The objective of this review is to analyse the sociodemographic disparities in ophthalmological phases III and IV CT based on publicly available data.
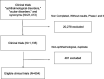
Methods: This study included phases III and IV complete ophthalmological CT available from clinicaltrials.org, and describes the country distribution, race and ethnicity description and gender, and funding characteristics.
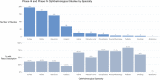
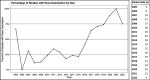
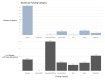
Results: After a screening process, we included 654 CTs, with findings that corroborate the previous CT reviews' findings that most ophthalmological participants are white and from high-income countries. A description of race and ethnicity is reported in 37.1% of studies but less frequently included within the most studied ophthalmological specialty area (cornea, retina, glaucoma and cataracts). The incidence of race and ethnicity reporting has improved during the past 7 years.
Discussion: Although NIH and FDA promote guidelines to improve generalisability in healthcare studies, the inclusion of race and ethnicity in publications and diverse participants in ophthalmological CT is still limited. Actions from the research community and related stakeholders are necessary to increase representativeness and guarantee generalisability in ophthalmological research results to optimise care and reduce related healthcare disparities.
Keywords: Clinical Trial; Epidemiology; Public health.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Racial/Ethnic Disparities in Ophthalmology Clinical Trials Resulting in US Food and Drug Administration Drug Approvals From 2000 to 2020.JAMA Ophthalmol. 2021 Jun 1;139(6):629-637. doi: 10.1001/jamaophthalmol.2021.0857. JAMA Ophthalmol. 2021. PMID: 33885724 Free PMC article.
-
[Increase in examinations for cataracts, glaucoma, diabetic retinopathy and age-related macular degeneration : Comparative cross-sectional study between 2010 and 1997 in ophthalmological practices].Ophthalmologe. 2014 Aug;111(8):757-64. doi: 10.1007/s00347-013-2966-z. Ophthalmologe. 2014. PMID: 24343245 German.
-
Reporting results in U.S. clinical trials for obstructive sleep apnea and insomnia: How transparent are they?Sleep Health. 2020 Aug;6(4):529-533. doi: 10.1016/j.sleh.2019.11.009. Epub 2020 Mar 14. Sleep Health. 2020. PMID: 32179065 Free PMC article. Review.
-
A global review of publicly available datasets for ophthalmological imaging: barriers to access, usability, and generalisability.Lancet Digit Health. 2021 Jan;3(1):e51-e66. doi: 10.1016/S2589-7500(20)30240-5. Epub 2020 Oct 1. Lancet Digit Health. 2021. PMID: 33735069 Review.
-
Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 May 3;4(5):e218348. doi: 10.1001/jamanetworkopen.2021.8348. JAMA Netw Open. 2021. PMID: 34003274 Free PMC article.
Cited by
-
Gender and ethnic diversity in randomised clinical trials in age-related macular degeneration and diabetic macular oedema.Eye (Lond). 2025 May;39(7):1249-1253. doi: 10.1038/s41433-025-03595-7. Epub 2025 Feb 20. Eye (Lond). 2025. PMID: 39979609 Free PMC article. Review.
-
A Practical Guide to Evaluating Artificial Intelligence Imaging Models in Scientific Literature.Ophthalmol Sci. 2025 Jun 9;5(6):100847. doi: 10.1016/j.xops.2025.100847. eCollection 2025 Nov-Dec. Ophthalmol Sci. 2025. PMID: 40778360 Free PMC article.
-
Inequities in glaucoma research: an analysis of Cochrane systematic reviews and randomized trials.J Clin Epidemiol. 2025 May;181:111717. doi: 10.1016/j.jclinepi.2025.111717. Epub 2025 Feb 8. J Clin Epidemiol. 2025. PMID: 39929324
References
-
- NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Available: https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm [Accessed 20 Jun 2022].
-
- Office of the Commissioner . FDA’S action plan for FDASIA section 907: questions and answers. U.S. food and drug administration. Available: https://www.fda.gov/regulatory-information/food-and-drug-administration-...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
