Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease
- PMID: 37278488
- PMCID: PMC10243114
- DOI: 10.1002/14651858.CD013532.pub2
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease
Abstract
Background: Advanced chronic liver disease is characterised by a long compensated phase followed by a rapidly progressive 'decompensated' phase, which is marked by the development of complications of portal hypertension and liver dysfunction. Advanced chronic liver disease is considered responsible for more than one million deaths annually worldwide. No treatment is available to specifically target fibrosis and cirrhosis; liver transplantation remains the only curative option. Researchers are investigating strategies to restore liver functionality to avoid or slow progression towards end-stage liver disease. Cytokine mobilisation of stem cells from the bone marrow to the liver could improve liver function. Granulocyte colony-stimulating factor (G-CSF) is a 175-amino-acid protein currently available for mobilisation of haematopoietic stem cells from the bone marrow. Multiple courses of G-CSF, with or without stem or progenitor cell or growth factors (erythropoietin or growth hormone) infusion, might be associated with accelerated hepatic regeneration, improved liver function, and survival.
Objectives: To evaluate the benefits and harms of G-CSF with or without stem or progenitor cell or growth factors (erythropoietin or growth hormone) infusion, compared with no intervention or placebo in people with compensated or decompensated advanced chronic liver disease.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, three other databases, and two trial registers (October 2022) together with reference-checking and web-searching to identify additional studies. We applied no restrictions on language and document type.
Selection criteria: We only included randomised clinical trials comparing G-CSF, independent of the schedule of administration, as a single treatment or combined with stem or progenitor cell infusion, or with other medical co-interventions, with no intervention or placebo, in adults with chronic compensated or decompensated advanced chronic liver disease or acute-on-chronic liver failure. We included trials irrespective of publication type, publication status, outcomes reported, or language.
Data collection and analysis: We followed standard Cochrane procedures. All-cause mortality, serious adverse events, and health-related quality of life were our primary outcomes, and liver disease-related morbidity, non-serious adverse events, and no improvement of liver function scores were our secondary outcomes. We undertook meta-analyses, based on intention-to-treat, and presented results using risk ratios (RR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, with 95% confidence intervals (CI) and I2 statistic values as a marker of heterogeneity. We assessed all outcomes at maximum follow-up. We determined the certainty of evidence using GRADE, evaluated the risk of small-study effects in regression analyses, and conducted subgroup and sensitivity analyses.
Main results: We included 20 trials (1419 participants; sample size ranged from 28 to 259), which lasted between 11 and 57 months. Nineteen trials included only participants with decompensated cirrhosis; in one trial, 30% had compensated cirrhosis. The included trials were conducted in Asia (15), Europe (four), and the USA (one). Not all trials provided data for our outcomes. All trials reported data allowing intention-to-treat analyses. The experimental intervention consisted of G-CSF alone or G-CSF plus any of the following: growth hormone, erythropoietin, N-acetyl cysteine, infusion of CD133-positive haemopoietic stem cells, or infusion of autologous bone marrow mononuclear cells. The control group consisted of no intervention in 15 trials and placebo (normal saline) in five trials. Standard medical therapy (antivirals, alcohol abstinence, nutrition, diuretics, β-blockers, selective intestinal decontamination, pentoxifylline, prednisolone, and other supportive measures depending on the clinical status and requirement) was administered equally to the trial groups. Very low-certainty evidence suggested a decrease in mortality with G-CSF, administered alone or in combination with any of the above, versus placebo (RR 0.53, 95% CI 0.38 to 0.72; I2 = 75%; 1419 participants; 20 trials). Very low-certainty evidence suggested no difference in serious adverse events (G-CSF alone or in combination versus placebo: RR 1.03, 95% CI 0.66 to 1.61; I2 = 66%; 315 participants; three trials). Eight trials, with 518 participants, reported no serious adverse events. Two trials, with 165 participants, used two components of the quality of life score for assessment, with ranges from 0 to 100, where higher scores indicate better quality of life, with a mean increase from baseline of the physical component summary of 20.7 (95% CI 17.4 to 24.0; very low-certainty evidence) and a mean increase from baseline of the mental component summary of 27.8 (95% CI 12.3 to 43.3; very low-certainty evidence). G-CSF, alone or in combination, suggested a beneficial effect on the proportion of participants who developed one or more liver disease-related complications (RR 0.40, 95% CI 0.17 to 0.92; I2 = 62%; 195 participants; four trials; very low-certainty evidence). When we analysed the occurrences of single complications, there was no suggestion of a difference between G-CSF, alone or in combination, versus control, in participants in need of liver transplantation (RR 0.85, 95% CI 0.39 to 1.85; 692 participants; five trials), in the development of hepatorenal syndrome (RR 0.65, 95% CI 0.33 to 1.30; 520 participants; six trials), in the occurrence of variceal bleeding (RR 0.68, 95% CI 0.37 to 1.23; 614 participants; eight trials), and in the development of encephalopathy (RR 0.56, 95% CI 0.31 to 1.01; 605 participants; seven trials) (very low-certainty evidence). The same comparison suggested that G-CSF reduces the development of infections (including sepsis) (RR 0.50, 95% CI 0.29 to 0.84; 583 participants; eight trials) and does not improve liver function scores (RR 0.67, 95% CI 0.53 to 0.86; 319 participants; two trials) (very low-certainty evidence).
Authors' conclusions: G-CSF, alone or in combination, seems to decrease mortality in people with decompensated advanced chronic liver disease of whatever aetiology and with or without acute-on-chronic liver failure, but the certainty of evidence is very low because of high risk of bias, inconsistency, and imprecision. The results of trials conducted in Asia and Europe were discrepant; this could not be explained by differences in participant selection, intervention, and outcome measurement. Data on serious adverse events and health-related quality of life were few and inconsistently reported. The evidence is also very uncertain regarding the occurrence of one or more liver disease-related complications. We lack high-quality, global randomised clinical trials assessing the effect of G-CSF on clinically relevant outcomes.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AC: has declared that they have no conflict of interest. MF: has declared that they have no conflict of interest. DP: has declared that they have no conflict of interest. GC: has declared that they have no conflict of interest.
Figures
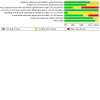

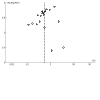






















Update of
- doi: 10.1002/14651858.CD013532
Similar articles
-
Gene therapy for people with hepatocellular carcinoma.Cochrane Database Syst Rev. 2024 Jun 4;6(6):CD013731. doi: 10.1002/14651858.CD013731.pub2. Cochrane Database Syst Rev. 2024. PMID: 38837373 Free PMC article.
-
Tamoxifen for adults with hepatocellular carcinoma.Cochrane Database Syst Rev. 2024 Aug 12;8(8):CD014869. doi: 10.1002/14651858.CD014869.pub2. Cochrane Database Syst Rev. 2024. PMID: 39132750 Free PMC article.
-
Corticosteroids for treatment of leptospirosis.Cochrane Database Syst Rev. 2025 Jul 24;7(7):CD014935. doi: 10.1002/14651858.CD014935.pub2. Cochrane Database Syst Rev. 2025. PMID: 40704556
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis.Cochrane Database Syst Rev. 2018 May 15;5(5):CD012410. doi: 10.1002/14651858.CD012410.pub2. Cochrane Database Syst Rev. 2018. PMID: 29762873 Free PMC article.
Cited by
-
Characterization of inflammatory protein expression patterns and their association with viral DNA load in hepatitis B virus infection via Olink proteomics analysis.Am J Transl Res. 2025 Jun 15;17(6):4701-4712. doi: 10.62347/JTXH6594. eCollection 2025. Am J Transl Res. 2025. PMID: 40672613 Free PMC article.
-
Hepatorenal Syndrome-Novel Insights into Diagnostics and Treatment.Int J Mol Sci. 2023 Dec 14;24(24):17469. doi: 10.3390/ijms242417469. Int J Mol Sci. 2023. PMID: 38139297 Free PMC article. Review.
-
Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis.J Clin Med. 2023 Oct 16;12(20):6541. doi: 10.3390/jcm12206541. J Clin Med. 2023. PMID: 37892679 Free PMC article. Review.
References
References to studies included in this review
De 2021 {published data only}
-
- De A, Kumari S, Singh A, Kaur A, Sharma R, Bhalla A, et al. Multiple cycles of granulocyte colony-stimulating factor increase survival times of patients with decompensated cirrhosis in a randomized trial. Clinical Gastroenterology and Hepatology 2021;19:375-83. - PubMed
Duan 2013 {published data only}
Engelmann 2021a {published data only}
-
- Engelmann C, Herber A, Franke A, Bruns T, Reuken P, Schiefke I, et al. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: a multicenter randomized trial (GRAFT study). Journal of Hepatology 2021;75(6):1346-54. - PubMed
Garg 2012 {published data only}
-
- Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142(3):505-12. - PubMed
Haque 2020 {published data only}
Kedarisetty 2015 {published data only}
-
- Kedarisetty CK, Anand L, Bhardwaj A, Bhadoria AS, Kumar G, Vyas AK, et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 2015;148(7):1362-70. - PubMed
Morgan 2022 {published data only}
Newsome 2018 {published data only}
-
- Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet. Gastroenterology & Hepatology 2018;3(1):25-36. - PMC - PubMed
Prajapati 2017 {published data only}
-
- Prajapati R, Arora A, Sharma P, Bansal N, Singla V, Kumar A. Granulocyte colony-stimulating factor improves survival of patients with decompensated cirrhosis: a randomized-controlled trial. European Journal of Gastroenterology & Hepatology 2017;29:448-55. - PubMed
Saha 2017 {published data only}
-
- Saha BK, Mahtab MA, Akbar SMF, Noor-E-Alam SM, Mamun AA, Hossain SM, et al. APASL ACLF working party. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: increased survival and containment of liver damage. Hepatology International 2017;11(6):540-6. - PubMed
Sharma 2017 {published data only}
-
- Sharma A, Setia A, Rai RR. Effect of granulocyte colony-stimulating factor (G-CSF) on mortality and complications viz. sepsis, encephalopathy, hepatorenal syndrome, and gastrointestinal bleed in severe alcoholic hepatitis: a randomized controlled study. United European Gastroenterology Journal 2017;5:A17.
Shasthry 2019 {published data only}
-
- Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology 2019;70(3):802-11. - PubMed
Singh 2014 {published data only}
-
- Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. American Journal of Gastroenterology 2014;109(9):1417-23. - PubMed
Singh 2018b {published data only}
-
- Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, et al. Efficacy of granulocyte colony-stimulating factor and n-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clinical Gastroenterology and Hepatology 2018;16(10):1650-6. - PubMed
Singh 2021 {published data only}
-
- Singh V, Singh A, Sharma N, De A, Leishangthem B, Sharma N, et al. Granulocyte-colony stimulating factor (G-CSF) with or without four weeks of n-acetyl cysteine (NAC) in severe alcoholic hepatitis. Hepatology 2021;Suppl 1:215A.
Spahr 2008 {published data only}
-
- Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48(1):221-9. - PubMed
Spahr 2013 {published data only}
Tong 2022 {published data only}
Venkitaraman 2022 {published data only}
Verma 2018a {published data only}
-
- Verma N, Kaur A, Sharma R, Bhalla A, Sharma N, De A, et al. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology 2018;68(4):1559-73. - PubMed
References to studies excluded from this review
Amer 2011 {published data only}
-
- Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. European Journal of Gastroenterology & Hepatology 2011;23(10):936-41. - PubMed
Anand 2019 {published data only}
-
- Anand L, Bihari C, Kedarisetty CK, Rooge SB, Kumar D, Shubham S, et al. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver International 2019;39(1):115-26. - PubMed
El‐Ansary 2012 {published data only}
-
- El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Reviews and Reports 2012;8(3):972-81. - PubMed
Esmaeilzadeh 2019 {published data only}
Fiuza 2002 {published data only}
Gaia 2013 {published data only}
-
- Gaia S, Olivero A, Smedile A, Ruella M, Abate ML, Fadda M, et al. Multiple courses of G-CSF in patients with decompensated cirrhosis: consistent mobilization of immature cells expressing hepatocyte markers and exploratory clinical evaluation. Hepatology International 2013;7(4):1075-83. - PubMed
Kharaziha 2009 {published data only}
-
- Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. European Journal of Gastroenterology & Hepatology 2009;21(10):1199-205. - PubMed
Lyra 2010 {published data only}
-
- Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. European Journal of Gastroenterology & Hepatology 2010;22(1):33-42. - PubMed
Mohamadnejad 2016 {published data only}
Philips 2020 {published data only}
Ranjan 2022 {published data only}
-
- Ranjan A, Sarin SK, Maiwall R, Jindal A, Rajan V. Comparison of imipenem and tigecycline versus imipenem, tigecycline combined with GM-CSF in patients with spontaneous bacterial peritonitis and septic shock. Journal of Clinical and Experimental Hepatology 2022;12(Suppl 2):S26.
Sakaida 2005 {published data only}
-
- Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Medical Molecular Morphology 2005;38(4):197-202. - PubMed
Salama 2010 {published data only}
-
- Salama H, Zekri AR, Zern M, Bahnassy A, Loutfy S, Shalaby S, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplantation 2010;19:1475-86. - PubMed
Sharma 2016 {published data only}
-
- Sharma V, Sharma R, Rao P. Exciting results with injection darbepoietin alfa and pegfilgrastim in patients with decompensated liver cirrhosis. Journal of Liver Research, Disorders & Therapy 2016;2(5):131-2.
Singh 2018a {published data only}
Terai 2006 {published data only}
-
- Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006;24(10):2292-8. - PubMed
Xing 2013 {published data only}
-
- Xing TJ, Xu HT, Xian JC, Shen ML, Li H, Ye J, et al. Mechanism and efficacy of mobilization of granulocyte colony-stimulating factor in the treatment of chronic hepatic failure. Hepatogastroenterology 2013;60(121):170-5. - PubMed
Yannaki 2005 {published data only}
-
- Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Experimental Hematology 2005;33(1):108-19. - PubMed
References to studies awaiting assessment
Xu 2016 {published data only}
-
- Xu X, Liu XY, Chen J, Xiao L, Tong JJ, Guan CD, et al. Randomised controlled clinical study of granulocyte colony-stimulating factor in the treatment of chronic hepatitis B-related acute liver failure. Infectious Diseases Information 2016;29(5):279-83.
Zhou 2020 {published data only}
-
- Zhou P, Yu Z, Qv SY. The clinical trial of G-CSF for end-stage alcoholic liver disease. Chinese Hepatology 2020;25:521-4.
References to ongoing studies
Cho 2018 {published data only}
Additional references
Alison 2000
-
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000;406:257. - PubMed
Arroyo 2017
-
- Arroyo V, Moreau R. Diagnosis and prognosis of acute on chronic liver failure (ACLF) in cirrhosis. Journal of Hepatology 2017;66:451-3. - PubMed
Arroyo 2020
-
- Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. New England Journal of Medicine 2020;382(22):2137-45. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. - PubMed
Castellini 2018
Chavez‐Tapia 2015
-
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, et al. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Annals of Hepatology 2015;14(5):631-41. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988.
D'Amico 2006
-
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44:217-31. - PubMed
D'Amico 2014
-
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39:1180-93. - PubMed
de Franchis 2015
-
- Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63:743-52. - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341-50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. - PubMed
Duong 2014
-
- Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation 2014;20:1262-73. - PubMed
EASL 2018
-
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406-60. - PubMed
Engelmann 2021b
-
- Engelmann C, Martino VD, Kerbert AJ, Weil-Verhoeven D, Aehling NF, Herber A, et al. The current status of granulocyte-colony stimulating factor to treat acute-on-chronic liver failure. Seminars in Liver Disease 2021;41(3):298-307. - PubMed
Forbes 2012
-
- Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. Journal of Hepatology 2012;56:496-9. - PubMed
Forbes 2016
-
- Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nature Reviews. Gastroenterology and Hepatology 2016;13:473-85. - PubMed
Foucher 2006
Garattini 2016
-
- Garattini S, Jakobsen JC, Wetterslev J, Bertele V, Banzi R, Rath A, et al. Evidence-based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13-21. - PubMed
Garcia‐Tsao 2010
Gartlehner 2019
-
- Gartlehner G, Nussbaumer-Streit B, Wagner G, Patel S, Swinson-Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2019;106:50-9. - PubMed
Gilchrist 2010
-
- Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transplantation 2010;16:118-29. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 4 December 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395-400. - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311-6. - PubMed
Guyatt 2011h
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. - PubMed
Guyatt 2013c
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables - continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173-83. - PubMed
Guyatt 2013d
-
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719-25. - PubMed
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. - PubMed
Harbord 2006
Higgins 2011a
Higgins 2011b
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated August 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. - PMC - PubMed
Higgins 2019b
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Hou 2021
Huang 2021
ICH‐GCP 2016
-
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 16 June 2022).
Jakobsen 2014
King 2015
-
- King A, Barton D, Beard HA, N Than, Moore J, Corbett C, et al. REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open 2015;5:e007700. - PMC - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(1):982-9. - PubMed
Krupczak‐Hollis 2003
-
- Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology 2003;38(6):1552-62. - PubMed
Lanthier 2018
-
- Lanthier N. Haemopoietic stem cell therapy in cirrhosis: the end of the story? Lancet. Gastroenterology & Hepatology 2018;3(1):3-5. - PubMed
Lewis 2004
-
- Lewis LD. Preclinical and clinical studies: a preview of potential future applications of erythropoietic agents. Seminars in Hematology 2004;41(Suppl 7):17-25. - PubMed
Li 2019
-
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Lundh 2017
Marot 2020
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. - PubMed
Moore 2014
-
- Moore K, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Alimentary Pharmacology & Therapeutics 2014;39:673-85. - PubMed
Murray 2013
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42; quiz 742.e1-5. - PubMed
NICE 2016
-
- NICE Guideline [NG50]. Cirrhosis in over 16s: assessment and management. www.nice.org.uk/guidance/ng50 (accessed 9 November 2022).
Page 2022
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Pavlov 2015
-
- Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD010542. [DOI: 10.1002/14651858.CD010542.pub2] - DOI - PMC - PubMed
Pimpin 2018
-
- Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. Journal of Hepatology 2018;69(3):718-35. - PubMed
Review Manager [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rossi 2007
Rowe 2017
-
- Rowe IA. Lessons from epidemiology: the burden of liver disease. Digestive Diseases 2017;35:304-9. - PubMed
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27:746-63. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1-82. - PubMed
Savović 2018
Schultz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408-12. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2022
-
- Shi P, Zhang J, Wu M, Zheng T, Liang A, Wen Z, et al. The effects of granulocyte-colony stimulating factor on chronic liver disease: a meta-analysis. Journal of Infection in Developing Countries 2022;16(3):537-46. - PubMed
Storebø 2018
-
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] - DOI - PMC - PubMed
Thomas 2011
-
- Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration and function. Hepatology 2011;53:2003-15. - PubMed
Thorlund 2017
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more (accessed 16 June 2022).
TSA [Computer program]
-
- TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017. Available at ctu.dk/tsa/downloads/.
Tsochatzis 2014
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. - PubMed
Verma 2018b
-
- Verma N, Singh A, Singh V. Reply. Hepatology 2018;68(1):388. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. - PubMed
Wetterslev 2009
Wetterslev 2017
Wood 2008
References to other published versions of this review
Colli 2020
-
- Colli A, Prati D, Fraquelli M, Casazza G. Granulocyte colony‐stimulating factor with or without stem or progenitor cell infusion for people with compensated or decompensated advanced chronic liver disease. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013532. [DOI: 10.1002/14651858.CD013532] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

