The generation of HepG2 transmitochondrial cybrids to reveal the role of mitochondrial genotype in idiosyncratic drug-induced liver injury
- PMID: 37278519
- PMCID: PMC10270688
- DOI: 10.7554/eLife.78187
The generation of HepG2 transmitochondrial cybrids to reveal the role of mitochondrial genotype in idiosyncratic drug-induced liver injury
Abstract
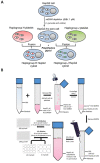
Background: Evidence supports an important link between mitochondrial DNA (mtDNA) variation and adverse drug reactions such as idiosyncratic drug-induced liver injury (iDILI). Here, we describe the generation of HepG2-derived transmitochondrial cybrids, to investigate the impact of mtDNA variation on mitochondrial (dys)function and susceptibility to iDILI. This study created 10 cybrid cell lines, each containing distinct mitochondrial genotypes of haplogroup H or haplogroup J backgrounds.
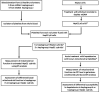
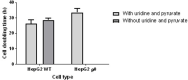
Methods: HepG2 cells were depleted of mtDNA to make rho zero cells, before the introduction of known mitochondrial genotypes using platelets from healthy volunteers (n=10), thus generating 10 transmitochondrial cybrid cell lines. The mitochondrial function of each was assessed at basal state and following treatment with compounds associated with iDILI; flutamide, 2-hydroxyflutamide, and tolcapone, and their less toxic counterparts bicalutamide and entacapone utilizing ATP assays and extracellular flux analysis.
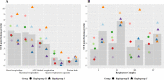
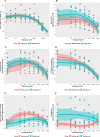
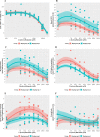
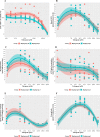
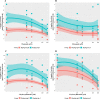
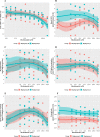
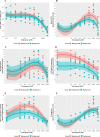
Results: Whilst only slight variations in basal mitochondrial function were observed between haplogroups H and J, haplogroup-specific responses were observed to the mitotoxic drugs. Haplogroup J showed increased susceptibility to inhibition by flutamide, 2-hydroxyflutamide, and tolcapone, via effects on selected mitochondrial complexes (I and II), and an uncoupling of the respiratory chain.
Conclusions: This study demonstrates that HepG2 transmitochondrial cybrids can be created to contain the mitochondrial genotype of any individual of interest. This provides a practical and reproducible system to investigate the cellular consequences of variation in the mitochondrial genome, against a constant nuclear background. Additionally, the results show that inter-individual variation in mitochondrial haplogroup may be a factor in determining sensitivity to mitochondrial toxicants.
Funding: This work was supported by the Centre for Drug Safety Science supported by the Medical Research Council, United Kingdom (Grant Number G0700654); and GlaxoSmithKline as part of an MRC-CASE studentship (grant number MR/L006758/1).
Keywords: HepG2; drug safety; drug-induced liver injury; human; medicine; mitochondria; mtDNA; transmitochondrial cybrid.
© 2023, Ball et al.
Conflict of interest statement
AB, AA holds a grant from GlaxoSmithKline (GSK) for this work. The author has no other competing interests to declare, CJ salary funded by Janssen Pharmaceutical, paid to University of Liverpool. The author has no other competing interests to declare, ML is an employee of GlaxoSmithKline (GSK), JL is an employee of GlaxoSmithKline (GSK). Unpaid role as a member of the Investigative Toxicology Leaders Forum (ITLF) representing GSK on this group. Provides unpaid consultation to Cambridge University in areas of drug development. The author has no other competing interests to declare, AC holds a grant from GlaxoSmithKline (GSK) for this work and received other funding from Janssen in 2017-2020 (paid directly to University of Liverpool). The author has no other competing interests to declare
Figures

Update of
- doi: 10.1101/2022.03.21.485109
Similar articles
-
Assessment of the impact of mitochondrial genotype upon drug-induced mitochondrial dysfunction in platelets derived from healthy volunteers.Arch Toxicol. 2021 Apr;95(4):1335-1347. doi: 10.1007/s00204-021-02988-3. Epub 2021 Feb 13. Arch Toxicol. 2021. PMID: 33585966 Free PMC article.
-
Differential mitochondrial and cellular responses between H vs. J mtDNA haplogroup-containing human RPE transmitochondrial cybrid cells.Exp Eye Res. 2022 Jun;219:109013. doi: 10.1016/j.exer.2022.109013. Epub 2022 Mar 10. Exp Eye Res. 2022. PMID: 35283109 Free PMC article.
-
The creation of cybrids harboring mitochondrial haplogroups in the Taiwanese population of ethnic Chinese background: an extensive in vitro tool for the study of mitochondrial genomic variations.Oxid Med Cell Longev. 2012;2012:824275. doi: 10.1155/2012/824275. Epub 2012 Dec 6. Oxid Med Cell Longev. 2012. PMID: 23304256 Free PMC article.
-
Investigating the importance of individual mitochondrial genotype in susceptibility to drug-induced toxicity.Biochem Soc Trans. 2020 Jun 30;48(3):787-797. doi: 10.1042/BST20190233. Biochem Soc Trans. 2020. PMID: 32453388 Free PMC article. Review.
-
Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies.Redox Biol. 2014;2:619-31. doi: 10.1016/j.redox.2014.03.006. Epub 2014 Apr 1. Redox Biol. 2014. PMID: 25460729 Free PMC article. Review.
Cited by
-
Preclinical models of mitochondrial dysfunction: mtDNA and nuclear-encoded regulators in diverse pathologies.Front Aging. 2025 Jun 23;6:1585508. doi: 10.3389/fragi.2025.1585508. eCollection 2025. Front Aging. 2025. PMID: 40625678 Free PMC article. Review.
References
-
- Alfirevic A, Gonzalez-Galarza F, Bell C, Martinsson K, Platt V, Bretland G, Evely J, Lichtenfels M, Cederbrant K, French N, Naisbitt D, Park BK, Jones AR, Pirmohamed M. In Silico analysis of HLA associations with drug-induced liver injury: use of a HLA-genotyped DNA Archive from healthy volunteers. Genome Medicine. 2012;4:51. doi: 10.1186/gm350. - DOI - PMC - PubMed
-
- Bale SS, Vernetti L, Senutovitch N, Jindal R, Hegde M, Gough A, McCarty WJ, Bakan A, Bhushan A, Shun TY, Golberg I, DeBiasio R, Usta BO, Taylor DL, Yarmush ML. In vitro platforms for evaluating liver toxicity. Experimental Biology and Medicine. 2014;239:1180–1191. doi: 10.1177/1535370214531872. - DOI - PMC - PubMed
-
- Ball AL, Bloch KM, Rainbow L, Liu X, Kenny J, Lyon JJ, Gregory R, Alfirevic A, Chadwick AE. Assessment of the impact of mitochondrial genotype upon drug-induced mitochondrial dysfunction in platelets derived from healthy volunteers. Archives of Toxicology. 2021;95:1335–1347. doi: 10.1007/s00204-021-02988-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

