Ultrasound-stimulated microbubbles enhanced vascular disruption in fractionated radiotherapy-treated tumours via ASMase activation
- PMID: 37278613
- PMCID: PMC10259839
- DOI: 10.1242/dmm.049531
Ultrasound-stimulated microbubbles enhanced vascular disruption in fractionated radiotherapy-treated tumours via ASMase activation
Abstract
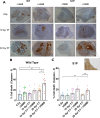
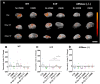
Recent studies have indicated that radiotherapy affects tumour vasculature as well as tumour cells. The use of ultrasound-stimulated microbubbles (USMB) can potentially enhance the effects of radiotherapy through the activation of the acid sphingomyelinase [ASMase or sphingomyelin phosphodiesterase 1 (SMPD1)]-ceramide pathway. ASMase knockout (ASMase-/-) and wild-type (WT) mice bearing fibrosarcoma (MCA/129 tumour line) were treated with 10 Gy or 20 Gy in five fractions alongside or independently of USMB treatments. The results indicated that tumour responses to fractionated radiotherapy (fXRT) were enhanced when fXRT was coupled with USMB as part of the treatment regimen. Sphingosine-1-phosphate (S1P)-treated mice and ASMase-/- mice demonstrated radioresistance against fXRT alone, whereas only ASMase-/- mice showed radioresistance against fXRT treatment alone and when combined with USMB. Results indicated that in WT and S1P-treated cohorts, the use of USMB with fXRT enhanced the tumour response compared to use of USMB or fXRT alone. Although in WT and S1P-treated cohorts, there was enhanced vascular disruption, ASMase-/- cohorts demonstrated no significant vascular disruption, indicating the importance of ASMase in facilitating vascular changes in response to fXRT and USMB treatment.
Keywords: Focused ultrasound; Fractionated radiation therapy; PC3 xenograft; Ultrasound stimulated microbubble therapy.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures






Similar articles
-
Role of Acid Sphingomyelinase and Ceramide in Mechano-Acoustic Enhancement of Tumor Radiation Responses.J Natl Cancer Inst. 2018 Sep 1;110(9):1009-1018. doi: 10.1093/jnci/djy011. J Natl Cancer Inst. 2018. PMID: 29506145 Free PMC article.
-
Ultrasound-stimulated microbubbles enhancement of fractionated radiation for tumor treatment.BMC Cancer. 2023 Jul 24;23(1):693. doi: 10.1186/s12885-023-10981-5. BMC Cancer. 2023. PMID: 37488490 Free PMC article.
-
Ultrasound-Based Radiation Enhancement: Concepts, Mechanisms and Therapeutic Applications.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241298864. doi: 10.1177/15330338241298864. Technol Cancer Res Treat. 2024. PMID: 39540206 Free PMC article.
-
Radiotherapeutic enhancement using ultrasound-stimulated microbubbles: a critical review.Int J Radiat Biol. 2025;101(7):664-684. doi: 10.1080/09553002.2025.2498980. Epub 2025 May 21. Int J Radiat Biol. 2025. PMID: 40397648 Review.
-
Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases.Cochrane Database Syst Rev. 2012 Apr 18;2012(4):CD003869. doi: 10.1002/14651858.CD003869.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 Jan 25;1:CD003869. doi: 10.1002/14651858.CD003869.pub4. PMID: 22513917 Free PMC article. Updated.
Cited by
-
Focused ultrasound-induced cell apoptosis for the treatment of tumours.PeerJ. 2024 Aug 21;12:e17886. doi: 10.7717/peerj.17886. eCollection 2024. PeerJ. 2024. PMID: 39184389 Free PMC article. Review.
-
Progression in low-intensity ultrasound-induced tumor radiosensitization.Cancer Med. 2024 Jul;13(13):e7332. doi: 10.1002/cam4.7332. Cancer Med. 2024. PMID: 38967145 Free PMC article. Review.
-
Comment in reply to Chen et al. Journal of Neuro-Oncology (2023) 165:535-545 "Focused ultrasound combined with radiotherapy for malignant brain tumor: a preclinical and clinical study".J Neurooncol. 2024 Jun;168(2):377-378. doi: 10.1007/s11060-024-04675-6. Epub 2024 Apr 19. J Neurooncol. 2024. PMID: 38639855 No abstract available.
-
Radiation enhancement using focussed ultrasound-stimulated microbubbles for breast cancer: A Phase 1 clinical trial.PLoS Med. 2024 May 17;21(5):e1004408. doi: 10.1371/journal.pmed.1004408. eCollection 2024 May. PLoS Med. 2024. PMID: 38758967 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

