Ultrafast single-molecule imaging reveals focal adhesion nano-architecture and molecular dynamics
- PMID: 37278764
- PMCID: PMC10244807
- DOI: 10.1083/jcb.202110162
Ultrafast single-molecule imaging reveals focal adhesion nano-architecture and molecular dynamics
Abstract
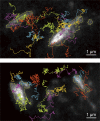
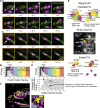
Using our newly developed ultrafast camera described in the companion paper, we reduced the data acquisition periods required for photoactivation/photoconversion localization microscopy (PALM, using mEos3.2) and direct stochastic reconstruction microscopy (dSTORM, using HMSiR) by a factor of ≈30 compared with standard methods, for much greater view-fields, with localization precisions of 29 and 19 nm, respectively, thus opening up previously inaccessible spatiotemporal scales to cell biology research. Simultaneous two-color PALM-dSTORM and PALM-ultrafast (10 kHz) single fluorescent-molecule imaging-tracking has been realized. They revealed the dynamic nanoorganization of the focal adhesion (FA), leading to the compartmentalized archipelago FA model, consisting of FA-protein islands with broad diversities in size (13-100 nm; mean island diameter ≈30 nm), protein copy numbers, compositions, and stoichiometries, which dot the partitioned fluid membrane (74-nm compartments in the FA vs. 109-nm compartments outside the FA). Integrins are recruited to these islands by hop diffusion. The FA-protein islands form loose ≈320 nm clusters and function as units for recruiting FA proteins.
© 2023 Fujiwara et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








Comment in
-
Ultra high-speed single-molecule fluorescence imaging.J Cell Biol. 2023 Aug 7;222(8):e202306136. doi: 10.1083/jcb.202306136. Epub 2023 Jul 17. J Cell Biol. 2023. PMID: 37458726 Free PMC article.

