Nanopore Filter: A Method for Counting and Extracting Single DNA Molecules Using a Biological Nanopore
- PMID: 37279035
- PMCID: PMC10797584
- DOI: 10.1021/acs.analchem.3c00573
Nanopore Filter: A Method for Counting and Extracting Single DNA Molecules Using a Biological Nanopore
Abstract
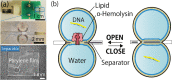
This paper describes a method for the real-time counting and extraction of DNA molecules at the single-molecule level by nanopore technology. As a powerful tool for electrochemical single-molecule detection, nanopore technology eliminates the need for labeling or partitioning sample solutions at the femtoliter level. Here, we attempt to develop a DNA filtering system utilizing an α-hemolysin (αHL) nanopore. This system comprises two droplets, one filling with and one emptying DNA molecules, separated by a planar lipid bilayer containing αHL nanopores. The translocation of DNA through the nanopores is observed by measuring the channel current, and the number of translocated molecules can also be verified by quantitative polymerase chain reaction (qPCR). However, we found that the issue of contamination seems to be an almost insolvable problem in single-molecule counting. To tackle this problem, we tried to optimize the experimental environment, reduce the volume of solution containing the target molecule, and use the PCR clamp method. Although further efforts are still needed to achieve a single-molecule filter with electrical counting, our proposed method shows a linear relationship between the electrical counting and qPCR estimation of the number of DNA molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

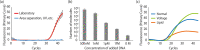


Similar articles
-
Lipid bilayer coated Al(2)O(3) nanopore sensors: towards a hybrid biological solid-state nanopore.Biomed Microdevices. 2011 Aug;13(4):671-82. doi: 10.1007/s10544-011-9537-3. Biomed Microdevices. 2011. PMID: 21487665 Free PMC article.
-
Nanoscale Probing of Informational Polymers with Nanopores. Applications to Amyloidogenic Fragments, Peptides, and DNA-PNA Hybrids.Acc Chem Res. 2019 Jan 15;52(1):267-276. doi: 10.1021/acs.accounts.8b00565. Epub 2019 Jan 3. Acc Chem Res. 2019. PMID: 30605305
-
Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores.Nat Nanotechnol. 2010 Dec;5(12):874-7. doi: 10.1038/nnano.2010.237. Epub 2010 Nov 28. Nat Nanotechnol. 2010. PMID: 21113160 Free PMC article.
-
Biological nanopores for sensing applications.Proteins. 2022 Oct;90(10):1786-1799. doi: 10.1002/prot.26308. Epub 2022 Feb 9. Proteins. 2022. PMID: 35092317 Review.
-
Peering into biological nanopore: a practical technology to single-molecule analysis.Chem Asian J. 2010 Sep 3;5(9):1952-61. doi: 10.1002/asia.201000279. Chem Asian J. 2010. PMID: 20669216 Review.
Cited by
-
Lipid vesicle-based molecular robots.Lab Chip. 2024 Feb 27;24(5):996-1029. doi: 10.1039/d3lc00860f. Lab Chip. 2024. PMID: 38239102 Free PMC article. Review.
-
Redesign of Translocon EXP2 Nanopore for Detecting Peptide Fragments.Small Methods. 2025 Apr;9(4):e2401562. doi: 10.1002/smtd.202401562. Epub 2025 Feb 5. Small Methods. 2025. PMID: 39905884 Free PMC article.
References
-
- Denuga S.; Whelan D. E.; O’Neill S. P.; Johnson R. P. Capture and analysis of double-stranded DNA with the α-hemolysin nanopore: Fundamentals and applications. Electrochem. Sci. Adv. 2022, 2, e220000110.1002/elsa.202200001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

