Alveolar repair following LPS-induced injury requires cell-ECM interactions
- PMID: 37279065
- PMCID: PMC10443799
- DOI: 10.1172/jci.insight.167211
Alveolar repair following LPS-induced injury requires cell-ECM interactions
Abstract
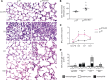
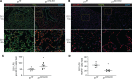
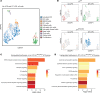
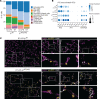
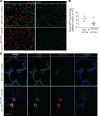
During alveolar repair, alveolar type 2 (AT2) epithelial cell progenitors rapidly proliferate and differentiate into flat AT1 epithelial cells. Failure of normal alveolar repair mechanisms can lead to loss of alveolar structure (emphysema) or development of fibrosis, depending on the type and severity of injury. To test if β1-containing integrins are required during repair following acute injury, we administered E. coli lipopolysaccharide (LPS) by intratracheal injection to mice with a postdevelopmental deletion of β1 integrin in AT2 cells. While control mice recovered from LPS injury without structural abnormalities, β1-deficient mice had more severe inflammation and developed emphysema. In addition, recovering alveoli were repopulated with an abundance of rounded epithelial cells coexpressing AT2 epithelial, AT1 epithelial, and mixed intermediate cell state markers, with few mature type 1 cells. AT2 cells deficient in β1 showed persistently increased proliferation after injury, which was blocked by inhibiting NF-κB activation in these cells. Lineage tracing experiments revealed that β1-deficient AT2 cells failed to differentiate into mature AT1 epithelial cells. Together, these findings demonstrate that functional alveolar repair after injury with terminal alveolar epithelial differentiation requires β1-containing integrins.
Keywords: Cell Biology; Integrins; Pulmonology; Respiration.
Conflict of interest statement
Figures



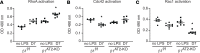

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL157373/HL/NHLBI NIH HHS/United States
- R01 HL150617/HL/NHLBI NIH HHS/United States
- P01 HL092870/HL/NHLBI NIH HHS/United States
- K08 DK134879/DK/NIDDK NIH HHS/United States
- K08 HL143051/HL/NHLBI NIH HHS/United States
- R01 HL168556/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- L40 HL138848/HL/NHLBI NIH HHS/United States
- R03 HL154287/HL/NHLBI NIH HHS/United States
- R01 DK127589/DK/NIDDK NIH HHS/United States
- R01 HL163195/HL/NHLBI NIH HHS/United States
- R01 DK088327/DK/NIDDK NIH HHS/United States
- R01 HL153246/HL/NHLBI NIH HHS/United States
- K08 HL127102/HL/NHLBI NIH HHS/United States
- R38 HL143619/HL/NHLBI NIH HHS/United States
- R01 DK069921/DK/NIDDK NIH HHS/United States
- T32 GM152284/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

