Platelets exacerbate cardiovascular inflammation in a murine model of Kawasaki disease vasculitis
- PMID: 37279077
- PMCID: PMC10443810
- DOI: 10.1172/jci.insight.169855
Platelets exacerbate cardiovascular inflammation in a murine model of Kawasaki disease vasculitis
Abstract
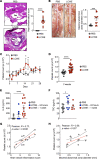
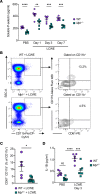
Kawasaki disease (KD) is the leading cause of acquired heart disease among children. Increased platelet counts and activation are observed during the course of KD, and elevated platelet counts are associated with higher risks of developing intravenous immunoglobulin resistance and coronary artery aneurysms. However, the role of platelets in KD pathogenesis remains unclear. Here, we analyzed transcriptomics data generated from the whole blood of patients with KD and discovered changes in the expression of platelet-related genes during acute KD. In the Lactobacillus casei cell wall extract (LCWE) murine model of KD vasculitis, LCWE injection increased platelet counts and the formation of monocyte-platelet aggregates (MPAs), upregulated the concentration of soluble P-selectin, and increased circulating thrombopoietin and interleukin 6 (IL-6). Furthermore, platelet counts correlated with the severity of cardiovascular inflammation. Genetic depletion of platelets (Mpl-/- mice) or treatment with an anti-CD42b antibody significantly reduced LCWE-induced cardiovascular lesions. Furthermore, in the mouse model, platelets promoted vascular inflammation via the formation of MPAs, which likely amplified IL-1B production. Altogether, our results indicate that platelet activation exacerbates the development of cardiovascular lesions in a murine model of KD vasculitis. These findings enhance our understanding of KD vasculitis pathogenesis and highlight MPAs, which are known to enhance IL-1B production, as a potential therapeutic target for this disorder.
Keywords: Cardiovascular disease; Inflammation; Platelets; Vascular Biology; Vasculitis.
Figures



Comment in
-
Platelets promote cardiovascular complications in Kawasaki disease.Nat Rev Rheumatol. 2023 Sep;19(9):537. doi: 10.1038/s41584-023-01011-6. Nat Rev Rheumatol. 2023. PMID: 37532781 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

