Adavosertib Enhances Antitumor Activity of Trastuzumab Deruxtecan in HER2-Expressing Cancers
- PMID: 37279095
- PMCID: PMC10618648
- DOI: 10.1158/1078-0432.CCR-23-0103
Adavosertib Enhances Antitumor Activity of Trastuzumab Deruxtecan in HER2-Expressing Cancers
Abstract
Purpose: Cyclin E (CCNE1) has been proposed as a biomarker of sensitivity to adavosertib, a Wee1 kinase inhibitor, and a mechanism of resistance to HER2-targeted therapy.
Experimental design: Copy number and genomic sequencing data from The Cancer Genome Atlas and MD Anderson Cancer Center databases were analyzed to assess ERBB2 and CCNE1 expression. Molecular characteristics of tumors and patient-derived xenografts (PDX) were assessed by next-generation sequencing, whole-exome sequencing, fluorescent in situ hybridization, and IHC. In vitro, CCNE1 was overexpressed or knocked down in HER2+ cell lines to evaluate drug combination efficacy. In vivo, NSG mice bearing PDXs were subjected to combinatorial therapy with various treatment regimens, followed by tumor growth assessment. Pharmacodynamic markers in PDXs were characterized by IHC and reverse-phase protein array.
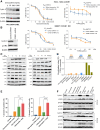
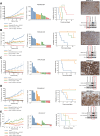
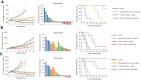
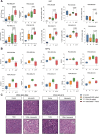
Results: Among several ERBB2-amplified cancers, CCNE1 co-amplification was identified (gastric 37%, endometroid 43%, and ovarian serous adenocarcinoma 41%). We hypothesized that adavosertib may enhance activity of HER2 antibody-drug conjugate trastuzumab deruxtecan (T-DXd). In vitro, sensitivity to T-DXd was decreased by cyclin E overexpression and increased by knockdown, and adavosertib was synergistic with topoisomerase I inhibitor DXd. In vivo, the T-DXd + adavosertib combination significantly increased γH2AX and antitumor activity in HER2 low, cyclin E amplified gastroesophageal cancer PDX models and prolonged event-free survival (EFS) in a HER2-overexpressing gastroesophageal cancer model. T-DXd + adavosertib treatment also increased EFS in other HER2-expressing tumor types, including a T-DXd-treated colon cancer model.
Conclusions: We provide rationale for combining T-DXd with adavosertib in HER2-expressing cancers, especially with co-occuring CCNE1 amplifications. See related commentary by Rolfo et al., p. 4317.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
Comment in
-
Empower the Potential of Trastuzumab Deruxtecan with Novel Combinations.Clin Cancer Res. 2023 Nov 1;29(21):4317-4319. doi: 10.1158/1078-0432.CCR-23-1700. Clin Cancer Res. 2023. PMID: 37656059
References
-
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology 2001;61Suppl 2:1–13. - PubMed
-
- Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003;22:6570–8. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. - PubMed
-
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

