ZBTB20 is essential for cochlear maturation and hearing in mice
- PMID: 37279265
- PMCID: PMC10268240
- DOI: 10.1073/pnas.2220867120
ZBTB20 is essential for cochlear maturation and hearing in mice
Abstract
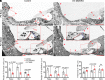
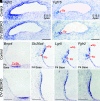
The mammalian cochlear epithelium undergoes substantial remodeling and maturation before the onset of hearing. However, very little is known about the transcriptional network governing cochlear late-stage maturation and particularly the differentiation of its lateral nonsensory region. Here, we establish ZBTB20 as an essential transcription factor required for cochlear terminal differentiation and maturation and hearing. ZBTB20 is abundantly expressed in the developing and mature cochlear nonsensory epithelial cells, with transient expression in immature hair cells and spiral ganglion neurons. Otocyst-specific deletion of Zbtb20 causes profound deafness with reduced endolymph potential in mice. The subtypes of cochlear epithelial cells are normally generated, but their postnatal development is arrested in the absence of ZBTB20, as manifested by an immature appearance of the organ of Corti, malformation of tectorial membrane (TM), a flattened spiral prominence (SP), and a lack of identifiable Boettcher cells. Furthermore, these defects are related with a failure in the terminal differentiation of the nonsensory epithelium covering the outer border Claudius cells, outer sulcus root cells, and SP epithelial cells. Transcriptome analysis shows that ZBTB20 regulates genes encoding for TM proteins in the greater epithelial ridge, and those preferentially expressed in root cells and SP epithelium. Our results point to ZBTB20 as an essential regulator for postnatal cochlear maturation and particularly for the terminal differentiation of cochlear lateral nonsensory domain.
Keywords: auditory; cell differentiation; cochlea; hearing; transcription factor.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo.J Neurosci. 2003 Jun 1;23(11):4395-400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. J Neurosci. 2003. PMID: 12805278 Free PMC article.
-
Expression of Atoh1, Gfi1, and Pou4f3 in the mature cochlea reprograms nonsensory cells into hair cells.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2304680121. doi: 10.1073/pnas.2304680121. Epub 2024 Jan 24. Proc Natl Acad Sci U S A. 2024. PMID: 38266052 Free PMC article.
-
LDLR expression in the cochlea suggests a role in endolymph homeostasis and cochlear amplification.Hear Res. 2021 Sep 15;409:108311. doi: 10.1016/j.heares.2021.108311. Epub 2021 Jul 15. Hear Res. 2021. PMID: 34311268
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration.Hear Res. 2013 Mar;297:68-83. doi: 10.1016/j.heares.2012.11.009. Epub 2012 Nov 16. Hear Res. 2013. PMID: 23164734 Free PMC article. Review.
Cited by
-
Lmx1a is essential for marginal cell differentiation and stria vascularis formation.Front Cell Dev Biol. 2025 Mar 5;13:1537505. doi: 10.3389/fcell.2025.1537505. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40109362 Free PMC article.
-
Baseline SUVmax is correlated with tumor hypoxia and patient outcomes in nasopharyngeal carcinoma.Sci Rep. 2024 Aug 30;14(1):20157. doi: 10.1038/s41598-024-71191-y. Sci Rep. 2024. PMID: 39215035 Free PMC article.
-
The role of ATP-binding Cassette subfamily B member 6 in the inner ear.Nat Commun. 2024 Nov 18;15(1):9885. doi: 10.1038/s41467-024-53663-x. Nat Commun. 2024. PMID: 39557842 Free PMC article.
-
Cochlear Implantation in Primrose Syndrome with a Novel ZBTB20 Gene Variant.Turk Arch Otorhinolaryngol. 2023 Dec;61(4):192-200. doi: 10.4274/tao.2023.2023-4-5. Epub 2024 May 21. Turk Arch Otorhinolaryngol. 2023. PMID: 38784957 Free PMC article.
-
Activated tissue-resident macrophages contribute to hair cell insults in noise-induced hearing loss in mice.Commun Biol. 2024 Sep 2;7(1):1078. doi: 10.1038/s42003-024-06768-4. Commun Biol. 2024. PMID: 39223249 Free PMC article.
References
-
- Lim D. J., Anniko M., Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol. Suppl. 422, 1–69 (1985). - PubMed
-
- Roth B., Bruns V., Postnatal development of the rat organ of Corti. I. General morphology, basilar membrane, tectorial membrane and border cells. Anat. Embryol. (Berl) 185, 559–569 (1992). - PubMed
-
- Jagger D. J., Forge A., The enigmatic root cell–emerging roles contributing to fluid homeostasis within the cochlear outer sulcus. Hear. Res. 303, 1–11 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

