Gene expression detection in developing mouse tissue using in situ hybridization and µCT imaging
- PMID: 37279266
- PMCID: PMC10268296
- DOI: 10.1073/pnas.2301876120
Gene expression detection in developing mouse tissue using in situ hybridization and µCT imaging
Abstract
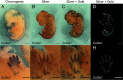
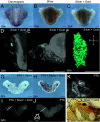
High resolution and noninvasiveness have made soft-tissue X-ray microtomography (µCT) a widely applicable three-dimensional (3D) imaging method in studies of morphology and development. However, scarcity of molecular probes to visualize gene activity with µCT has remained a challenge. Here, we apply horseradish peroxidase-assisted reduction of silver and catalytic gold enhancement of the silver deposit to in situ hybridization in order to detect gene expression in developing tissues with µCT (here called GECT, gene expression CT). We show that GECT detects expression patterns of collagen type II alpha 1 and sonic hedgehog in developing mouse tissues comparably with an alkaline phosphatase-based detection method. After detection, expression patterns are visualized with laboratory µCT, demonstrating that GECT is compatible with varying levels of gene expression and varying sizes of expression regions. Additionally, we show that the method is compatible with prior phosphotungstic acid staining, a conventional contrast staining approach in µCT imaging of soft tissues. Overall, GECT is a method that can be integrated with existing laboratory routines to obtain spatially accurate 3D detection of gene expression.
Keywords: development; gene expression; in situ hybridization; µCT imaging.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Mizutani R., Suzuki Y., X-ray microtomography in biology. Micron 43, 104–115 (2012). - PubMed
-
- Pauwels E., Van Loo D., Cornillie P., Brabant L., Van Hoorebeke L., An exploratory study of contrast agents for soft tissue visualization by means of high resolution X-ray computed tomography imaging. J. Microsc. 250, 21–31 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

