Safety and Efficacy of Riluzole in Acute Spinal Cord Injury Study (RISCIS): A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial
- PMID: 37279301
- PMCID: PMC10460693
- DOI: 10.1089/neu.2023.0163
Safety and Efficacy of Riluzole in Acute Spinal Cord Injury Study (RISCIS): A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial
Abstract
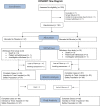
Riluzole is a sodium-glutamate antagonist that attenuates neurodegeneration in amyotrophic lateral sclerosis (ALS). It has shown favorable results in promoting recovery in pre-clinical models of traumatic spinal cord injury (tSCI) and in early phase clinical trials. This study aimed to evaluate the efficacy and safety of riluzole in acute cervical tSCI. An international, multi-center, prospective, randomized, double-blinded, placebo-controlled, adaptive, Phase III trial (NCT01597518) was undertaken. Patients with American Spinal Injury Association Impairment Scale (AIS) A-C, cervical (C4-C8) tSCI, and <12 h from injury were randomized to receive either riluzole, at an oral dose of 100 mg twice per day (BID) for the first 24 h followed by 50 mg BID for the following 13 days, or placebo. The primary efficacy end-point was change in Upper Extremity Motor (UEM) scores at 180 days. The primary efficacy analyses were conducted on an intention to treat (ITT) and completed cases (CC) basis. The study was powered at a planned enrolment of 351 patients. The trial began in October 2013 and was halted by the sponsor on May 2020 (and terminated in April 2021) in the face of the global COVID-19 pandemic. One hundred ninety-three patients (54.9% of the pre-planned enrolment) were randomized with a follow-up rate of 82.7% at 180 days. At 180 days, in the CC population the riluzole-treated patients compared with placebo had a mean gain of 1.76 UEM scores (95% confidence interval: -2.54-6.06) and 2.86 total motor scores (CI: -6.79-12.52). No drug-related serious adverse events were associated with the use of riluzole. Additional pre-planned sensitivity analyses revealed that in the AIS C population, riluzole was associated with significant improvement in total motor scores (estimate: standard error [SE] 8.0; CI 1.5-14.4) and upper extremity motor scores (SE 13.8; CI 3.1-24.5) at 6 months. AIS B patients had higher reported independence, measured by the Spinal Cord Independence Measure score (45.3 vs. 27.3; d: 18.0 CI: -1.7-38.0) and change in mental health scores, measured by the Short Form 36 mental health domain (2.01 vs. -11.58; d: 13.2 CI: 1.2-24.8) at 180 days. AIS A patients who received riluzole had a higher average gain in neurological levels at 6 months compared with placebo (mean 0.50 levels gained vs. 0.12 in placebo; d: 0.38, CI: -0.2-0.9). The primary analysis did not achieve the predetermined end-point of efficacy for riluzole, likely related to insufficient power. However, on pre-planned secondary analyses, all subgroups of cervical SCI subjects (AIS grades A, B and C) treated with riluzole showed significant gains in functional recovery. The results of this trial may warrant further investigation to extend these findings. Moreover, guideline development groups may wish to assess the possible clinical relevance of the secondary outcome analyses, in light of the fact that SCI is an uncommon orphan disorder without an accepted neuroprotective treatment.
Keywords: clinical trial; glutamate antagonist; neuroprotection; neurotrauma; sodium channel blocker; spinal cord injury.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury randomized controlled trial. J Am Med Assoc 1997;277(20):1597–1604. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
