Cationic proteins from eosinophils bind bone morphogenetic protein receptors promoting vascular calcification and atherogenesis
- PMID: 37279475
- PMCID: PMC10393071
- DOI: 10.1093/eurheartj/ehad262
Cationic proteins from eosinophils bind bone morphogenetic protein receptors promoting vascular calcification and atherogenesis
Abstract
Aims: Blood eosinophil count and eosinophil cationic protein (ECP) concentration are risk factors of cardiovascular diseases. This study tested whether and how eosinophils and ECP contribute to vascular calcification and atherogenesis.
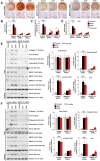
Methods and results: Immunostaining revealed eosinophil accumulation in human and mouse atherosclerotic lesions. Eosinophil deficiency in ΔdblGATA mice slowed atherogenesis with increased lesion smooth muscle cell (SMC) content and reduced calcification. This protection in ΔdblGATA mice was muted when mice received donor eosinophils from wild-type (WT), Il4-/-, and Il13-/- mice or mouse eosinophil-associated-ribonuclease-1 (mEar1), a murine homologue of ECP. Eosinophils or mEar1 but not interleukin (IL) 4 or IL13 increased the calcification of SMC from WT mice but not those from Runt-related transcription factor-2 (Runx2) knockout mice. Immunoblot analyses showed that eosinophils and mEar1 activated Smad-1/5/8 but did not affect Smad-2/3 activation or expression of bone morphogenetic protein receptors (BMPR-1A/1B/2) or transforming growth factor (TGF)-β receptors (TGFBR1/2) in SMC from WT and Runx2 knockout mice. Immunoprecipitation showed that mEar1 formed immune complexes with BMPR-1A/1B but not TGFBR1/2. Immunofluorescence double-staining, ligand binding, and Scatchard plot analysis demonstrated that mEar1 bound to BMPR-1A and BMPR-1B with similar affinity. Likewise, human ECP and eosinophil-derived neurotoxin (EDN) also bound to BMPR-1A/1B on human vascular SMC and promoted SMC osteogenic differentiation. In a cohort of 5864 men from the Danish Cardiovascular Screening trial and its subpopulation of 394 participants, blood eosinophil counts and ECP levels correlated with the calcification scores of different arterial segments from coronary arteries to iliac arteries.
Conclusion: Eosinophils release cationic proteins that can promote SMC calcification and atherogenesis using the BMPR-1A/1B-Smad-1/5/8-Runx2 signalling pathway.
Keywords: Bone morphogenetic protein receptors; Calcification; Eosinophil; Eosinophil cationic protein; Smooth muscle cell.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest All authors declare no conflict of interest for this contribution.
Figures








Comment in
-
Eosinophils promote vascular calcification and atherosclerosis: adding another layer of complexity on the path to clarity?Eur Heart J. 2023 Aug 1;44(29):2784-2786. doi: 10.1093/eurheartj/ehad323. Eur Heart J. 2023. PMID: 37282593 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL163099/HL/NHLBI NIH HHS/United States
- R01 HL134892/HL/NHLBI NIH HHS/United States
- I01 BX004426/BX/BLRD VA/United States
- HL151627/HL/NHLBI NIH HHS/United States
- AG063839/NH/NIH HHS/United States
- R01 HL136165/HL/NHLBI NIH HHS/United States
- R01 HL158097/HL/NHLBI NIH HHS/United States
- R01 HL151627/HL/NHLBI NIH HHS/United States
- R01 HL167201/HL/NHLBI NIH HHS/United States
- R01 AG063839/AG/NIA NIH HHS/United States
- R01 AG082839/AG/NIA NIH HHS/United States
- R01 HL157073/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

