A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes
- PMID: 37279523
- PMCID: PMC10573729
- DOI: 10.1093/cid/ciad335
A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes
Abstract
Background: Antimicrobial resistance (AMR) is undermining modern medicine, a problem compounded by bacterial adaptation to antibiotic pressures. Phages are viruses that infect bacteria. Their diversity and evolvability offer the prospect of their use as a therapeutic solution. Reported are outcomes of customized phage therapy for patients with difficult-to-treat antimicrobial resistant infections.
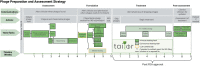
Methods: We retrospectively assessed 12 cases of customized phage therapy from a phage production center. Phages were screened, purified, sequenced, characterized, and Food and Drug Administration-approved via the IND (investigational new drug) compassionate-care route. Outcomes were assessed as favorable or unfavorable by microbiologic and clinical standards. Infections were device-related or systemic. Other experiences such as time to treatment, antibiotic synergy, and immune responses were recorded.
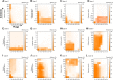
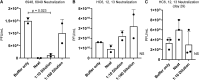
Results: Fifty requests for phage therapy were received. Customized phages were generated for 12 patients. After treatment, 42% (5/12) of cases showed bacterial eradication and 58% (7/12) showed clinical improvement, with two-thirds of all cases (66%) showing favorable responses. No major adverse reactions were observed. Antibiotic-phage synergy in vitro was observed in most cases. Immunological neutralization of phages was reported in 5 cases. Several cases were complicated by secondary infections. Complete characterization of the phages (morphology, genomics, and activity) and their production (methods, sterility, and endotoxin tests) are reported.
Conclusions: Customized phage production and therapy was safe and yielded favorable clinical or microbiological outcomes in two-thirds of cases. A center or pipeline dedicated to tailoring the phages against a patient's specific AMR bacterial infection may be a viable option where standard treatment has failed.
Keywords: antibiotic resistance; microbiology; phage; phage therapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. B. W. T. reports grants or contracts, all paid to their institution, from VA Health Services Research & Development; Agency for Healthcare Research and Quality (AHRQ) R18; Craig H. Neilsen Foundation; Genentech; Peptilogics, Inc; AHRQ R01; Center for Innovations in Quality, Effectiveness, and Safety (IQuESt) VA Health Services Research and Development (HSR&D) grant no. CIN 13-413, Bacteriophage to Treat Multidrug-Resistant UTI in Persons with Spinal Cord Injury (5I01RX002595-03); payment to the author for a George Washington ID Board Review Course; support for attending meetings and/or travel from the VA Office of Research and Development and Infectious Diseases Society of America (IDSA); unpaid participation on a Data and Safety Monitoring Board (DSMB) for Cooperative Studies Program #2004 VA. G. A. S. reports grants or contracts unrelated to this work from Adaptive Phage Therapeutics and Phagelux; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Wayne State University and SUNY Downstate; support for attending meetings and/or travel from Phage Futures, NIH/NIAID, International Committee of Military Medicine, University of Michigan, University of Pittsburgh, and Mayo Alumni Association; a leadership or fiduciary role on the Antimicrobial Resistance Leadership Group and Phagistry. S. A. reports research grants to their institution from Cystic Fibrosis Foundation, Armata Pharmaceuticals, Adaptive Phage Therapeutics, Contrafect, NIAID/NIH, and the National Center for Advancing Translational Sciences (NCATS)/NIH; consulting fees paid to the author from BioMx, and unpaid to the author from Phico; participation as a Medical Advisory Board member for Pherecydes Pharma; a role as an executive committee member for Infection Disease Community of Practice (unpaid) for the American Society of Transplantation and as Chair of the COVID-19 Task Force (unpaid) for the International Society of Heart and Lung Transplantation. S. B. D. reports grants or contracts to the University of California San Francisco (UCSF) from Gilead, Pfizer, F2G, Regeneron, Chan Zuckerberg Biohub, and NIAID/NIH; consulting fees to the author from Genentech and Janssen/J+J; support for travel to speak at IDWeek from the IDSA; patent US20100143379A1 for Mif agonists and antagonist and therapeutic uses thereof; a leadership or fiduciary role on the IDSA Antibacterial Resistance Committee, CADPH HAI Advisory Committee and Antibacterial Resistance Leadership Group Innovations Group, Laboratory Center, Mentorship Committee, Gram Positive Commitee, Immunosuppressed Host Group; payment to the author for clinical events committee/adjudication committee participation from Shinogi, Basilea, and Duke Clinical Research Institute. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- World Health Organization . Antimicrobial resistance. 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 1 October 2022.
-
- O’Neill J. Review on antimicrobial resistance antimicrobial resistance: tackling a crisis for the health and wealth of nations. London, 2014; Available at: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta.... Accessed 1 October 2022.

