Ebola Virus Disease Features Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome in the Rhesus Macaque Model
- PMID: 37279544
- PMCID: PMC10428198
- DOI: 10.1093/infdis/jiad203
Ebola Virus Disease Features Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome in the Rhesus Macaque Model
Abstract
Background: Ebola virus (EBOV) disease (EVD) is one of the most severe and fatal viral hemorrhagic fevers and appears to mimic many clinical and laboratory manifestations of hemophagocytic lymphohistiocytosis syndrome (HLS), also known as macrophage activation syndrome. However, a clear association is yet to be firmly established for effective host-targeted, immunomodulatory therapeutic approaches to improve outcomes in patients with severe EVD.
Methods: Twenty-four rhesus monkeys were exposed intramuscularly to the EBOV Kikwit isolate and euthanized at prescheduled time points or when they reached the end-stage disease criteria. Three additional monkeys were mock-exposed and used as uninfected controls.
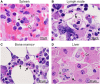
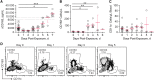
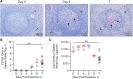
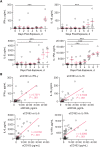
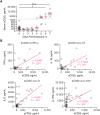
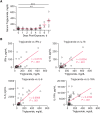
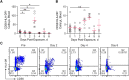
Results: EBOV-exposed monkeys presented with clinicopathologic features of HLS, including fever, multiple organomegaly, pancytopenia, hemophagocytosis, hyperfibrinogenemia with disseminated intravascular coagulation, hypertriglyceridemia, hypercytokinemia, increased concentrations of soluble CD163 and CD25 in serum, and the loss of activated natural killer cells.
Conclusions: Our data suggest that EVD in the rhesus macaque model mimics pathophysiologic features of HLS/macrophage activation syndrome. Hence, regulating inflammation and immune function might provide an effective treatment for controlling the pathogenesis of acute EVD.
Keywords: Ebola virus disease; hemophagocytic lymphohistiocytosis syndrome; macrophage activation syndrome; sCD163; sCD25.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2023.
Conflict of interest statement
Potential conflicts of interest . All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol 2018; 13:27–49. - PubMed
-
- Tan LH, Lum LC, Omar SF, Kan FK. Hemophagocytosis in dengue: comprehensive report of six cases. J Clin Virol 2012; 55:79–82. - PubMed
-
- Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. The pathogenesis of Ebola virus disease. Annu Rev Pathol 2017; 12:387–418. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

