Assessing the Impact of 2-Step Clostridioides difficile Testing at the Healthcare Facility Level
- PMID: 37279965
- PMCID: PMC10552580
- DOI: 10.1093/cid/ciad334
Assessing the Impact of 2-Step Clostridioides difficile Testing at the Healthcare Facility Level
Abstract
Background: Two-step testing for Clostridioides difficile infection (CDI) aims to improve diagnostic specificity but may also influence reported epidemiology and patterns of treatment. Some providers fear that 2-step testing may result in adverse outcomes if C. difficile is underdiagnosed.
Methods: Our primary objective was to assess the impact of 2-step testing on reported incidence of hospital-onset CDI (HO-CDI). As secondary objectives, we assessed the impact of 2-step testing on C. difficile-specific antibiotic use and colectomy rates as proxies for harm from underdiagnosis or delayed treatment. This longitudinal cohort study included 2 657 324 patient-days across 8 regional hospitals from July 2017 through March 2022. Impact of 2-step testing was assessed by time series analysis with generalized estimating equation regression models.
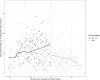
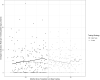
Results: Two-step testing was associated with a level decrease in HO-CDI incidence (incidence rate ratio, 0.53 [95% confidence interval {CI}, .48-.60]; P < .001), a similar level decrease in utilization rates for oral vancomycin and fidaxomicin (utilization rate ratio, 0.63 [95% CI, .58-.70]; P < .001), and no significant level (rate ratio, 1.16 [95% CI, .93-1.43]; P = .18) or trend (rate ratio, 0.85 [95% CI, .52-1.39]; P = .51) change in emergent colectomy rates.
Conclusions: Two-step testing is associated with decreased reported incidence of HO-CDI, likely by improving diagnostic specificity. The parallel decrease in C. difficile-specific antibiotic use offers indirect reassurance against underdiagnosis of C. difficile infections still requiring treatment by clinician assessment. Similarly, the absence of any significant change in colectomy rates offers indirect reassurance against any rise in fulminant C. difficile requiring surgical management.
Keywords: Clostridioides difficile; 2-step testing; interrupted time series analysis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. N. A. T. reports grant funding from the CDC, NIH/ARLG, and Rockefeller Foundation; research contract funding from PDI, Purio, and Basilea; and consulting fees for Techspert. D. J. A. reports grant funding from the CDC and Agency for Healthcare Research and Quality; contracts from CDC; interests as owner of Infection Control Education for Major Sports, LLC; and royalties from UpToDate. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Is the Healthcare Facility Level Sufficient for Assessing the Impact of 2-Step Clostridioides difficile Testing?Clin Infect Dis. 2023 Oct 5;77(7):1050-1052. doi: 10.1093/cid/ciad332. Clin Infect Dis. 2023. PMID: 37279961 No abstract available.
References
-
- Mawer D, Byrne F, Drake S, et al. . Cross-sectional study of the prevalence, causes and management of hospital-onset diarrhoea. J Hosp Infect 2019; 103:200–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

