Pharmacokinetic Data of Dolutegravir in Second-line Treatment of Children With Human Immunodeficiency Virus: Results From the CHAPAS4 Trial
- PMID: 37280040
- PMCID: PMC10640690
- DOI: 10.1093/cid/ciad346
Pharmacokinetic Data of Dolutegravir in Second-line Treatment of Children With Human Immunodeficiency Virus: Results From the CHAPAS4 Trial
Abstract
Background: Dolutegravir (DTG), combined with a backbone of 2 nucleoside reverse transcriptase inhibitors, is currently the preferred first-line treatment for human immunodeficiency virus (HIV) in childhood. CHAPAS4 is an ongoing randomized controlled trial investigating second-line treatment options for children with HIV. We did a nested pharmacokinetic (PK) substudy within CHAPAS4 to evaluate the DTG exposure in children with HIV taking DTG with food as part of their second-line treatment.
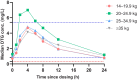
Methods: Additional consent was required for children on DTG enrolled in the CHAPAS4 trial to participate in this PK substudy. Children weighing 14-19.9 kg took 25 mg DTG as dispersible tablets and children ≥20 kg took 50 mg film-coated tablets. Steady-state 24-hour DTG plasma concentration-time PK profiling was done at t = 0 and 1, 2, 4, 6, 8, 12, and 24 hours after observed DTG intake with food. Reference adult PK data and pediatric data from the ODYSSEY trial were used primarily for comparison. The individual target trough concentration (Ctrough) was defined as 0.32 mg/L.
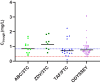
Results: Thirty-nine children on DTG were included in this PK substudy. The geometric mean (GM) area under the concentration-time curve over the dosing interval (AUC0-24h) was 57.1 hours × mg/L (coefficient of variation [CV%], 38.4%), which was approximately 8% below the average AUC0-24h in children in the ODYSSEY trial with comparable dosages, but above the adult reference. The GM (CV%) Ctrough was 0.82 mg/L (63.8%), which was comparable to ODYSSEY and adult reference values.
Conclusions: This nested PK substudy shows that the exposure of DTG taken with food in children on second-line treatment is comparable with that of children in the ODYSSEY trial and adult references. Clinical Trials Registration.ISRCTN22964075.
Keywords: DTG; HIV; children; pharmacokinetics; second-line.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . V. Mus. reports honorarium for speaking at a symposium from ViiV Healthcare; support to travel and attend an international conference from Viatris; and participation in an advisory board meeting and membership on a data and safety monitoring board (DSMB) for ViiV Healthcare. A. C. reports funding for trial paid to institution from ViiV Healthcare for Pharmacokinetics of newly developed ANtiretroviral agents in HIV-infected pregNAnt women (PANNA), Merck PANNA, and Gilead Sciences PANNA/UNIVERSAL relative bioavailability study; consulting fees from Gilead to institution; participation (no fee) on the Doravirine Dose Optimisation in Pregnancy (DORADO) independent DSMB (IDSMB) and Pharmacokinetics and Safety of DolutegravIr in Neonate (PETITE-DTG) IDSMB; and unpaid roles as co-chair of HIV, Hepatitis and STIs Pregnancy and Breastfeeding Therapeutics Working Group and member of Pediatric Antiretroviral Working Group for a World Health Organization (WHO) advisory group. D. B. reports grants or contracts and consulting fees paid to institution from Gilead Sciences, ViiV Healthcare, and Merck; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Pfizer, paid to institution; and participation on a DSMB or advisory board for Janssen, paid to institution. H. W. reports receiving consulting fees for an unrelated WHO project and consulting fees from European AIDS Clinical Society (EACS) as member of the guideline committee pediatric section of EACS HIV treatment guideline. V. Mul. reports EDCTP funds used for travel to research meetings and conferences (for EDCTP-funded studies), paid to institution – University of Zambia; and participation as member of the Zambia Medicines Regulatory Authority clinical trials technical review committee (fuel reimbursement for meetings attended). V. Mul. declares interest and does not attend review meetings for clinical trials that the author has participated on. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Joint United Nations Programme on HIV/AIDS . Young people and HIV.2021. Available at: https://www.unaids.org/sites/default/files/media_asset/young-people-and-.... Accessed 20 January 2023.
-
- Joint United Nations Programme on HIV/AIDS . Fact sheet 2022.2022. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_.... Accessed 20 January 2023.
-
- World Health Organization (WHO) . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring. Geneva, Switzerland: WHO, 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

