SARS-CoV-2 sero-immunity and quality of life in children and adolescents in relation to infections and vaccinations: the IMMUNEBRIDGE KIDS cross-sectional study, 2022
- PMID: 37280412
- PMCID: PMC10243264
- DOI: 10.1007/s15010-023-02052-5
SARS-CoV-2 sero-immunity and quality of life in children and adolescents in relation to infections and vaccinations: the IMMUNEBRIDGE KIDS cross-sectional study, 2022
Abstract
Purpose: The study evaluates the effects on sero-immunity, health status and quality of life of children and adolescents after the upsurge of the Omicron variant in Germany.
Methods: This multicenter cross-sectional study (IMMUNEBRIDGE Kids) was conducted within the German Network University Medicine (NUM) from July to October 2022. SARS-CoV-2- antibodies were measured and data on SARS-CoV-2 infections, vaccinations, health and socioeconomic factors as well as caregiver-reported evaluation on their children's health and psychological status were assessed.
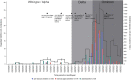
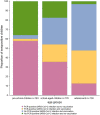
Results: 497 children aged 2-17 years were included. Three groups were analyzed: 183 pre-schoolchildren aged 2-4 years, 176 schoolchildren aged 5-11 years and 138 adolescents aged 12-18 years. Positive antibodies against the S- or N-antigen of SARS-CoV-2 were detected in 86.5% of all participants (70.0% [128/183] of pre-schoolchildren, 94.3% of schoolchildren [166/176] and 98.6% of adolescents [136/138]). Among all children, 40.4% (201/497) were vaccinated against COVID-19 (pre-schoolchildren 4.4% [8/183], schoolchildren 44.3% [78/176] and adolescents 83.3% [115/138]). SARS-CoV-2 seroprevalence was lowest in pre-school. Health status and quality of life reported by the parents were very positive at the time of the survey (Summer 2022).
Conclusion: Age-related differences on SARS-CoV-2 sero-immunity could mainly be explained by differences in vaccination rates based on the official German vaccination recommendations as well as differences in SARS-CoV-2 infection rates in the different age groups. Health status and quality of life of almost all children were very good independent of SARS-CoV-2 infection and/or vaccination.
Trial registration: German Registry for Clinical Trials Identifier Würzburg: DRKS00025546 (registration: 11.09.2021), Bochum: DRKS00022434 (registration:07.08.2020), Dresden: DRKS 00022455 (registration: 23.07.2020).
Keywords: Children and adolescents; Omicron; Quality of life; SARS-CoV-2; Seroprevalence.
© 2023. The Author(s).
Conflict of interest statement
Nicole Toepfner is a member of the directory board of the German Society for Pediatric Infectious Diseases (DGPI). Reinhard Berner is member of the Corona Expert Counsel of the German government, the Expert Advisory Board on pandemic Acute Respiratory Infections of the Robert Koch Institute, pandemic working group of the Standing committee on Vaccination (STIKO), medical pandemic counsel of the German Medical Association, scientific advisory board of the working group “long-COVID-syndrome” of the German Medical Association, task force Corona for the German Society of Pediatrics and Adolescent Medicine (DGKJ). Berit Lange has received the following grants: egePan Unimed (grant number 01KX2021), NUM COVIM 1.0 (grant number 01KX2021), PREPARED (grant number 01KX2021), RESPINOW (grant number MV2021-012), OptimAgent (grant number MV2021-014). Berit Lange is speaker for the “Modeling Network for Severe Infectious Diseases”, vice-president of the German Society of Epidemiology (DGEpi) and member of the internal advisory board of the German Center for Infection Research and member of the steering committee of TBNet. Andre Karch has received funding associated with the SARS-CoV-2 pandemic and other infectious epidemiology-related questions from the German Research Foundation, the German Federal Ministry of Education and Research, the German Federal Ministry of Health, the Robert Koch Institute, the “Landeszentrum Gesundheit Nordrhein-Westfalen”, “Niedersächsische Vorab” of the Volkswagen Foundation as well as innovation fund of the German federal Joint Committee.Hendrik Streeck has received fees for a discussion round considering “science communication” from Johnson&Johnson and is part of the scientific advisory board of AstraZeneca and Seqirus without receiving financial compensation. All other authors disclose no further conflicts of interests.
Figures
References
-
- Robert Koch Institut: Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19). https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsb.... Accessed 31.10.2022.
-
- Edward PR, Lorenzo-Redondo R, Reyna ME, Simons LM, Hultquist JF, Patel AB, et al. Severity of illness caused by severe acute respiratory syndrome coronavirus 2 variants of concern in children: a single-center retrospective cohort study. J Pediatric Infect Dis Soc. 2022;11:440–447. doi: 10.1093/jpids/piac068. - DOI - PMC - PubMed
-
- Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B. 1.1.5.29) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc. 2022;6:294–302. doi: 10.1016/S2352-4642(22)00027-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

