Correlated cryo-SEM and CryoNanoSIMS imaging of biological tissue
- PMID: 37280616
- PMCID: PMC10246362
- DOI: 10.1186/s12915-023-01623-0
Correlated cryo-SEM and CryoNanoSIMS imaging of biological tissue
Abstract
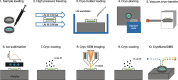
Background: The development of nanoscale secondary ion mass spectrometry (NanoSIMS) has revolutionized the study of biological tissues by enabling, e.g., the visualization and quantification of metabolic processes at subcellular length scales. However, the associated sample preparation methods all result in some degree of tissue morphology distortion and loss of soluble compounds. To overcome these limitations an entirely cryogenic sample preparation and imaging workflow is required.
Results: Here, we report the development of a CryoNanoSIMS instrument that can perform isotope imaging of both positive and negative secondary ions from flat block-face surfaces of vitrified biological tissues with a mass- and image resolution comparable to that of a conventional NanoSIMS. This capability is illustrated with nitrogen isotope as well as trace element mapping of freshwater hydrozoan Green Hydra tissue following uptake of 15N-enriched ammonium.
Conclusion: With a cryo-workflow that includes vitrification by high pressure freezing, cryo-planing of the sample surface, and cryo-SEM imaging, the CryoNanoSIMS enables correlative ultrastructure and isotopic or elemental imaging of biological tissues in their most pristine post-mortem state. This opens new horizons in the study of fundamental processes at the tissue- and (sub)cellular level.
Teaser: CryoNanoSIMS: subcellular mapping of chemical and isotopic compositions of biological tissues in their most pristine post-mortem state.
Keywords: High pressure freezing; Hydra viridissima; Isotope labeling; NanoSIMS; Osmoregulation; Photosymbiosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Slodzian G, Daigne B, Girard F, Hillion F. Secondary Ion Mass Spectrometry: SIMS IX. 1993. Ion optics for a high resolution scanning ion microscope and spectrometer: transmission evaluations; pp. 294–7.
-
- Hoppe P, Cohen S, Meibom A. NanoSIMS: Technical aspects and applications in cosmochemistry and biological geochemistry. Geostand Geoanal Res. 2013;37:111–154. doi: 10.1111/j.1751-908X.2013.00239.x. - DOI
-
- Zimmermann M, Escrig S, Hübschmann T, Kirf MK, Brand A, Inglis RF, et al. Phenotypic heterogeneity in metabolic traits among single cells of a rare bacterial species in its natural environment quantified with a combination of flow cell sorting and NanoSIMS. Front Microbiol. 2015;6:243. doi: 10.3389/fmicb.2015.00243. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

