Autoimmune Pancreatitis in Patients with Inflammatory Bowel Disease: A Real-World Multicentre Collaborative ECCO CONFER Study
- PMID: 37283545
- PMCID: PMC10673810
- DOI: 10.1093/ecco-jcc/jjad097
Autoimmune Pancreatitis in Patients with Inflammatory Bowel Disease: A Real-World Multicentre Collaborative ECCO CONFER Study
Erratum in
-
Correction to: Autoimmune Pancreatitis in Patients with Inflammatory Bowel Disease: A Real-World Multicentre Collaborative ECCO CONFER Study.J Crohns Colitis. 2025 May 8;19(5):jjaf077. doi: 10.1093/ecco-jcc/jjaf077. J Crohns Colitis. 2025. PMID: 40387533 Free PMC article. No abstract available.
Abstract
Background: Autoimmune pancreatitis [AIP] is rarely associated with inflammatory bowel disease [IBD]. The long-term outcomes of AIP and IBD in patients with coexisting AIP-IBD and predictors of complicated AIP course have rarely been reported.
Methods: An ECCO COllaborative Network For Exceptionally Rare case reports project [ECCO-CONFER] collected cases of AIP diagnosed in patients with IBD. Complicated AIP was defined as a composite of endocrine and/or exocrine pancreatic insufficiency, and/or pancreatic cancer. We explored factors associated with complicated AIP in IBD.
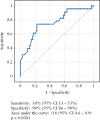
Results: We included 96 patients [53% males, 79% ulcerative colitis, 72% type 2 AIP, age at AIP diagnosis 35 ± 16 years]. The majority of Crohn's disease [CD] cases [78%] had colonic/ileocolonic involvement. In 59%, IBD preceded AIP diagnosis, whereas 18% were diagnosed simultaneously. Advanced therapy to control IBD was used in 61% and 17% underwent IBD-related surgery. In total, 82% of patients were treated with steroids for AIP, the majority of whom [91%] responded to a single course of treatment. During a mean follow-up of 7 years, AIP complications occurred in 25/96 [26%] individuals. In a multivariate model, older age at AIP diagnosis was associated with a complicated AIP course (odds ratio [OR] = 1.05, p = 0.008), whereas family history of IBD [OR = 0.1, p = 0.03], and CD diagnosis [OR = 0.2, p = 0.04] decreased the risk of AIP complications. No IBD- or AIP-related deaths occurred.
Conclusions: In this large international cohort of patients with concomitant AIP-IBD, most patients have type 2 AIP and colonic IBD. AIP course is relatively benign and long-term outcomes are favourable, but one-quarter develop pancreatic complications. Age, familial history of IBD, and CD may predict uncomplicated AIP course.
Keywords: Autoimmune pancreatitis; inflammatory bowel disease; pancreatic insufficiency.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
PE: Received lecture fees, consultancy fees, or travel educational grants from Takeda, Janssen, Pfizer, and Bristol Myers Squibb. BW: Research support from AbbVie, Biora Therapeutics, Pfizer, Sossei Heptares, and Takeda. Speaker’s fees from Abbvie, Biogen, Bristol Myers Squibb, Celltrion, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, Truvion, and Viatris. Consultancy fees from Abbvie, Alimentiv, Applied Strategic, Atheneum, Biora Therapeutics, Bristol Myers Squibb, Galapagos, Guidepont, Mylan, Inotrem, Ipsos, Janssen, Progenity, Sandoz, Sosei Heptares, Takeda, Tillots Pharma, and Viatris. EC: Consultancy fees from Zenus Biopharma, Horizon Therapeutics, Sanofi, Modern,a and Falk Pharma and is supported by the National Institute Health Research [NIHR] Oxford Biomedical Research Centre [BRC]. GD, LIK, JW, AGGP, MK, HL, MT, GO, DRG, TS, AB, JFR, DP, CFV, TJ, LL, SO, PP, IG, MJ, KM, NP, ABGS, ŁSz, RTW, YS, EZ, IM, DD, PEl: Declare no conflict of interest. TL: Grant support from Abbvie, Mylan, MSD, Mundipharma, Biogen, Janssen, Pfizer, and Takeda. Speaker’s fees from Ferring, MSD, Abbvie, Janssen, Amgen, Fresenius Kabi, and Takeda. Consultancy Fees: Janssen, Galapagos, Amgen, Bristol Myers Squibb, Fresenius Kab. CB: Received lecture fees and served as a consultant for Takeda, MSD, Ferring, Galapagos, and Janssen. PB: Financial support for research from Abbvie, Amgen, Celltrion, Mylan, Pfizer, and Takeda; advisory board fees from Abbvie, Arena pharmaceuticals, BMS, Celltrion, CIRC, Dr Falk, Galapagos, Janssen, Lilly, Pentax, PSI-CRO, Roche, Takeda, and Tetrameros; lecture fees from AbbVie, Celltrion, EPGS, Galapagos, Janssen, Lilly, Materia Prima, Pentax, Scope, and Takeda. KF: Received grant funding from the Crohn’s and Colitis Foundation. She serves on a GI Fellows Steering Committee for Janssen. MCh: Speaker’s consultancy, research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead, and Lilly. RW: Consultancy fee from Takeda, Janssen, and Abbvie. IGo: Research travel grants from ECCO and IOIBD. Institutional research support from Gilead, Boehringer Ingelheim, Pfizer, and Abbvie.