Vaccine Derived Poliovirus (VDPV)
- PMID: 37283637
- PMCID: PMC10241397
- DOI: 10.53854/liim-3102-5
Vaccine Derived Poliovirus (VDPV)
Abstract
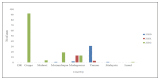
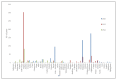
Poliomyelitis is caused by Poliovirus, a member of a large group of enteroviruses. Vaccine-derived polioviruses (VDPVs) stem from mutated live poliovirus, which is contained in the Oral Polio Virus vaccine (OPV). In addition, the emergence of VDPV is one of the global challenges for the eradication of poliomyelitis. VDPVs continue to affect different parts of the world; 1081 cases occurred in 2020 and 682 cases in 2021. There are several reasons that may have caused the increase in circulating vaccine-derived poliovirus (cVDPV) after the "switch" from the trivalent to the bivalent oral polio vaccine. One reason is the low vaccination rate among the targeted population, which has been further aggravated by the COVID-19 pandemic. Several strategies could control the spread of VDPV including the use of the monovalent OPV (mOPV-2). The risk of VDPV can be minimized through increased immunization rates and the use of safer vaccine alternatives. The global effort to eradicate polio has made significant progress over the years, but continued vigilance and investment in immunization programs are needed to achieve the ultimate goal of a polio-free world.
Keywords: Poliovirus; vaccine derivative poliovirus.
Conflict of interest statement
Conflict of interest None to declare.
Figures





References
-
- Global Polio Eradication Initiative. Classification and reporting of vaccine-derived polioviruses (VDPV) - GPEI guidelines 2016. [accessed February 22, 2023]. p. 8. http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Cla... .
-
- World Health Organization. Detection of circulating vaccine derived polio virus 2 (cVDPV2) in environmental samples - the United Kingdom of Great Britain and Northern Ireland and the United States of America. 2022. [accessed February 22, 2023]. https://www.who.int/emergencies/disease-out-break-news/item/2022-DON408 .
Publication types
LinkOut - more resources
Full Text Sources
