A Novel Mouse Model to Analyze Non-Genomic ERα Physiological Actions
- PMID: 37283844
- PMCID: PMC9338395
- DOI: 10.1210/jendso/bvac109
A Novel Mouse Model to Analyze Non-Genomic ERα Physiological Actions
Abstract
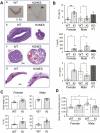
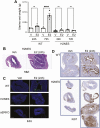
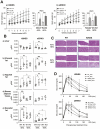
Nongenomic effects of estrogen receptor α (ERα) signaling have been described for decades. Several distinct animal models have been generated previously to analyze the nongenomic ERα signaling (eg, membrane-only ER, and ERαC451A). However, the mechanisms and physiological processes resulting solely from nongenomic signaling are still poorly understood. Herein, we describe a novel mouse model for analyzing nongenomic ERα actions named H2NES knock-in (KI). H2NES ERα possesses a nuclear export signal (NES) in the hinge region of ERα protein resulting in exclusive cytoplasmic localization that involves only the nongenomic action but not nuclear genomic actions. We generated H2NESKI mice by homologous recombination method and have characterized the phenotypes. H2NESKI homozygote mice possess almost identical phenotypes with ERα null mice except for the vascular activity on reendothelialization. We conclude that ERα-mediated nongenomic estrogenic signaling alone is insufficient to control most estrogen-mediated endocrine physiological responses; however, there could be some physiological responses that are nongenomic action dominant. H2NESKI mice have been deposited in the repository at Jax (stock no. 032176). These mice should be useful for analyzing nongenomic estrogenic responses and could expand analysis along with other ERα mutant mice lacking membrane-bound ERα. We expect the H2NESKI mouse model to aid our understanding of ERα-mediated nongenomic physiological responses and serve as an in vivo model for evaluating the nongenomic action of various estrogenic agents.
Keywords: estrogen; estrogen receptor alpha; extranuclear signaling; knock-in mutant mouse; nongenomic action.
Published by Oxford University Press on behalf of the Endocrine Society 2022.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
