Mutations, inflammation and phenotype of myeloproliferative neoplasms
- PMID: 37284191
- PMCID: PMC10239955
- DOI: 10.3389/fonc.2023.1196817
Mutations, inflammation and phenotype of myeloproliferative neoplasms
Abstract
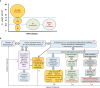
Knowledge on the myeloproliferative neoplasms (MPNs) - polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF) - has accumulated since the discovery of the JAK/STAT-activating mutations associated with MPNs: JAK2V617F, observed in PV, ET and PMF; and the MPL and CALR mutations, found in ET and PMF. The intriguing lack of disease specificity of these mutations, and of the chronic inflammation associated with MPNs, triggered a quest for finding what precisely determines that MPN patients develop a PV, ET or PMF phenoptype. The mechanisms of action of MPN-driving mutations, and concomitant mutations (ASXL1, DNMT3A, TET2, others), have been extensively studied, as well as the role played by these mutations in inflammation, and several pathogenic models have been proposed. In parallel, different types of drugs have been tested in MPNs (JAK inhibitors, interferons, hydroxyurea, anagrelide, azacytidine, combinations of those), some acting on both JAK2 and inflammation. Yet MPNs remain incurable diseases. This review aims to present current, detailed knowledge on the pathogenic mechanisms specifically associated with PV, ET or PMF that may pave the way for the development of novel, curative therapies.
Keywords: essential thrombocythemia inflammation; mutations; myeloproliferative neoplasms (MPN); polycythemia vera; primary fibrosis (PMF); therapeutic targets.
Copyright © 2023 Hermouet.
Conflict of interest statement
The author declares the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

