Association between immune-related adverse events and immunotherapy efficacy in non-small-cell lung cancer: a meta-analysis
- PMID: 37284302
- PMCID: PMC10239972
- DOI: 10.3389/fphar.2023.1190001
Association between immune-related adverse events and immunotherapy efficacy in non-small-cell lung cancer: a meta-analysis
Abstract
Objective: Our study aimed to identify potential correlations between anti-tumor efficacy and immune-related adverse events (irAEs) in non-small-cell lung cancer (NSCLC). Methods: We conducted a comprehensive search of online electronic databases up to March 2023 to identify any correlations between irAEs and immune checkpoint inhibitor (ICI) efficacy in NSCLC. We used meta-analysis RevMan 5.3 software to calculate pooled results. Results: Our meta-analysis of 54 studies revealed that patients who experienced irAEs achieved a significantly higher objective response rate (p < 0.00001) and longer progression-free survival (PFS) (p < 0.00001) and overall survival (OS) (p < 0.00001) than those who did not experience irAEs. Additionally, patients with ≥2 irAEs had better PFS, whereas no significant difference was observed between patients with or without squamous cell carcinoma. Subgroup analysis of irAE types indicated that irAEs (thyroid dysfunction and gastrointestinal, skin, or endocrine irAEs) were associated with better PFS and OS. However, no significant differences were observed between patients with pneumonitis or hepatobiliary irAEs. Conclusion: Our study showed that the occurrence of irAEs was a strong predictor of survival efficacy in patients with NSCLC treated with ICIs. Specifically, patients with ≥2 irAEs and those with thyroid dysfunction and gastrointestinal, skin, or endocrine irAEs achieved a better survival benefit. Systematic Review Registration: Website: https://www.crd.york.ac.uk/prospero/, Identifier: CRD42023421690.
Keywords: clinical efficacy; immune checkpoint inhibitors; immune-related adverse events; meta-analysis; non-small-cell lung cancer.
Copyright © 2023 Lin, Liu, Chen, Wei and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

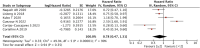



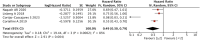

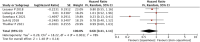


References
-
- Ahn B. C., Pyo K. H., Xin C. F., Jung D., Shim H. S., Lee C. Y., et al. (2019). Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J. cancer Res. Clin. Oncol. 145 (6), 1613–1623. 10.1007/s00432-019-02899-y - DOI - PMC - PubMed
-
- Akamatsu H., Murakami E., Oyanagi J., Shibaki R., Kaki T., Takase E., et al. (2020). Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncol. 25 (4), e679–e683. 10.1634/theoncologist.2019-0299 - DOI - PMC - PubMed
-
- Aso M., Toi Y., Sugisaka J., Aiba T., Kawana S., Saito R., et al. (2020). Association between skin reaction and clinical benefit in patients treated with anti-programmed cell death 1 monotherapy for advanced non-small cell lung cancer. Oncol. 25 (3), e536–e544. 10.1634/theoncologist.2019-0550 - DOI - PMC - PubMed
-
- Baldini E., Lunghi A., Cortesi E., Turci D., Signorelli D., Stati V., et al. (2020). Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung cancer 140, 59–64. 10.1016/j.lungcan.2019.12.014 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

