Puerarin inhibited oxidative stress and alleviated cerebral ischemia-reperfusion injury through PI3K/Akt/Nrf2 signaling pathway
- PMID: 37284311
- PMCID: PMC10240043
- DOI: 10.3389/fphar.2023.1134380
Puerarin inhibited oxidative stress and alleviated cerebral ischemia-reperfusion injury through PI3K/Akt/Nrf2 signaling pathway
Abstract
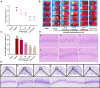
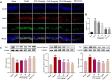
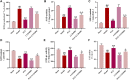
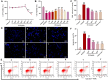
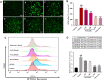
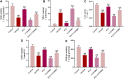
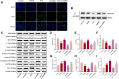
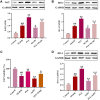
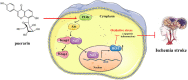
Introduction: Puerarin (PUE) is a natural compound isolated from Puerariae Lobatae Radix, which has a neuroprotective effect on IS. We explored the therapeutic effect and underlying mechanism of PUE on cerebral I/R injury by inhibiting oxidative stress related to the PI3K/Akt/Nrf2 pathway in vitro and in vivo. Methods: The middle cerebral artery occlusion and reperfusion (MCAO/R) rats and oxygen-glucose deprivation and reperfusion (OGD/R) were selected as the models, respectively. The therapeutic effect of PUE was observed using triphenyl tetrazolium and hematoxylin-eosin staining. Tunel-NeuN staining and Nissl staining to quantify hippocampal apoptosis. The reactive oxygen species (ROS) level was detected by flow cytometry and immunofluorescence. Biochemical method to detect oxidative stress levels. The protein expression related to PI3K/Akt/Nrf2 pathway was detected by using Western blotting. Finally, co-immunoprecipitation was used to study the molecular interaction between Keap1 and Nrf2. Results: In vivo and vitro studies showed that PUE improved neurological deficits in rats, as well as decreased oxidative stress. Immunofluorescence and flow cytometry indicated that the release of ROS can be inhibited by PUE. In addition, the Western blotting results showed that PUE promoted the phosphorylation of PI3K and Akt, and enabled Nrf2 to enter the nucleus, which further activated the expression of downstream antioxidant enzymes such as HO-1. The combination of PUE with PI3K inhibitor LY294002 reversed these results. Finally, co-immunoprecipitation results showed that PUE promoted Nrf2-Keap1 complex dissociation. Discussion: Taken together, PUE can activate Nrf2 via PI3K/Akt and promote downstream antioxidant enzyme expression, which could further ameliorate oxidative stress, against I/R-induced Neuron injury.
Keywords: PI3K/Akt/Nrf2 pathway; cerebral ischemia reperfusion injury (CIRI); hippocampal neurons; oxidative stress; puerarin.
Copyright © 2023 Zhang, Yao, Qi, Song, Wang, Li, Zhou, Chang, Huang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Puerarin alleviates cerebral ischemia/reperfusion (CIR)-induced neurocyte oxidative stress and apoptosis via DNA demethylation-mediated PI3K/Akt activation.Phytomedicine. 2025 Sep;145:157094. doi: 10.1016/j.phymed.2025.157094. Epub 2025 Jul 22. Phytomedicine. 2025. PMID: 40714421
-
HACE1 exerts a neuroprotective role against oxidative stress in cerebral ischemia-reperfusion injury by activating the PI3K/AKT/Nrf2 pathway.Neuroscience. 2024 Nov 1;559:249-262. doi: 10.1016/j.neuroscience.2024.09.002. Epub 2024 Sep 6. Neuroscience. 2024. PMID: 39244008
-
Echinocystic acid alleviated hypoxic-ischemic brain damage in neonatal mice by activating the PI3K/Akt/Nrf2 signaling pathway.Front Pharmacol. 2023 Feb 9;14:1103265. doi: 10.3389/fphar.2023.1103265. eCollection 2023. Front Pharmacol. 2023. PMID: 36843928 Free PMC article.
-
Neuroprotective effects of Chrysanthemum morifolium on cerebral ischemia- reperfusion injury contributes to the oxidative stress suppression and related Keap1/Nrf2 pathway.Brain Inj. 2023 Mar 21;37(4):269-281. doi: 10.1080/02699052.2022.2158225. Epub 2022 Dec 25. Brain Inj. 2023. PMID: 36567616
-
Nrf2-mediated therapeutic effects of dietary flavones in different diseases.Front Pharmacol. 2023 Sep 12;14:1240433. doi: 10.3389/fphar.2023.1240433. eCollection 2023. Front Pharmacol. 2023. PMID: 37767395 Free PMC article. Review.
Cited by
-
Mechanisms of mitophagy and oxidative stress in cerebral ischemia-reperfusion, vascular dementia, and Alzheimer's disease.Front Mol Neurosci. 2024 Aug 7;17:1394932. doi: 10.3389/fnmol.2024.1394932. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39169952 Free PMC article. Review.
-
PF4 regulates neuronal ferroptosis in cerebral hemorrhage through CXCR3/PI3K/AKT/Nrf2 pathway.Biomol Biomed. 2025 Apr 26;25(6):1322-1334. doi: 10.17305/bb.2024.11415. Biomol Biomed. 2025. PMID: 39601768 Free PMC article.
-
Hydrogel-mediated mitochondrial reprogramming for inducing cell death: a novel approach to overcoming gemcitabine resistance in pancreatic ductal adenocarcinoma.Mater Today Bio. 2025 Jun 1;32:101940. doi: 10.1016/j.mtbio.2025.101940. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40520559 Free PMC article.
-
Embryonic stem cell-derived small extracellular vesicles alleviate lead-induced cochlear spiral ganglion neurons injury by activating PI3 K/AKT signaling pathway.Sci Prog. 2025 Jul-Sep;108(3):368504251358007. doi: 10.1177/00368504251358007. Epub 2025 Jul 9. Sci Prog. 2025. PMID: 40630004 Free PMC article.
-
Puerarin protects renal ischemia-reperfusion injury in rats through NLRP3/Caspase-1/GSDMD pathway.Acta Cir Bras. 2023 Dec 1;38:e387323. doi: 10.1590/acb387323. eCollection 2023. Acta Cir Bras. 2023. PMID: 38055404 Free PMC article.
References
LinkOut - more resources
Full Text Sources

