Preventing swarm detection in extracellular vesicle flow cytometry: a clinically applicable procedure
- PMID: 37284418
- PMCID: PMC10239693
- DOI: 10.1016/j.rpth.2023.100171
Preventing swarm detection in extracellular vesicle flow cytometry: a clinically applicable procedure
Abstract
Background: Flow cytometry is commonly used to detect cell-derived extracellular vesicles in body fluids such as blood plasma. However, continuous and simultaneous illumination of multiple particles at or below the detection limit may result in the detection of a single event. This phenomenon is called swarm detection and leads to incorrect particle concentration measurements. To prevent swarm detection, sample dilution is recommended. Since the concentration of particles differs between plasma samples, finding the optimal sample dilution requires dilution series of all samples, which is unfeasible in clinical routine.
Objectives: Here we developed a practical procedure to find the optimal sample dilution of plasma for extracellular vesicle flow cytometry measurements in clinical research studies.
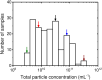
Methods: Dilution series of 5 plasma samples were measured with flow cytometry (Apogee A60-Micro), triggered on side scatter. The total particle concentration between these plasma samples ranged from 2.5 × 109 to 2.1 × 1011 mL-1.
Results: Swarm detection was absent in plasma samples when diluted ≥1.1 × 103-fold or at particle count rates <3.0 × 103 events·s-1. Application of either one of these criteria, however, resulted in insignificant particle counts in most samples. The best approach to prevent swarm detection while maintaining significant particle counts was by combining minimal dilution with maximum count rate.
Conclusion: To prevent swarm detection in a series of clinical samples, the measurement count rate of a single diluted plasma sample can be used to determine the optimal dilution factor. For our samples, flow cytometer, and settings, the optimal dilution factor is ≥1.1 × 102-fold, while the count rate is <1.1 × 104 events·s-1.
Keywords: biomarkers; exosomes; extracellular vesicles; flow cytometry; plasma.
© 2023 The Authors.
Figures



References
-
- van der Pol E., Sturk A., van Leeuwen T., Nieuwland R., Coumans F., ISTH-SSC-VB Working Group Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost. 2018;16:1236–1245. - PubMed
-
- Lacroix R., Robert S., Poncelet P., Kasthuri R.S., Key N.S., Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. - PubMed
-
- Nolan J.P., Duggan E. Analysis of individual extracellular vesicles by flow cytometry. Methods Mol Biol. 2018;1678:79–92. - PubMed
-
- Arraud N., Linares R., Tan S., Gounou C., Pasquet J.M., Mornet S., Brisson A.R. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–627. - PubMed
Associated data
LinkOut - more resources
Full Text Sources

