Equipping providers to offer novel MPTs: Developing counseling messages for the Dual Prevention Pill in clinical studies and beyond
- PMID: 37284490
- PMCID: PMC10239831
- DOI: 10.3389/frph.2023.1155948
Equipping providers to offer novel MPTs: Developing counseling messages for the Dual Prevention Pill in clinical studies and beyond
Abstract
Introduction: The pipeline for multi-purpose prevention technologies includes products that simultaneously prevent HIV, pregnancy and/or other sexually transmitted infections. Among these, the Dual Prevention Pill (DPP) is a daily pill co-formulating oral pre-exposure prophylaxis (PrEP), and combined oral contraception (COC). Clinical cross-over acceptability studies for the DPP require training providers to counsel on a combined product. From February 2021-April 2022, a working group of eight HIV and FP experts with clinical and implementation expertise developed counseling recommendations for the DPP based on existing PrEP/COC guidance.
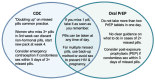
Assessment of policy/guidelines options and implications: The working group conducted a mapping of counseling messages from COC and oral PrEP guidance and provider training materials. Six topics were prioritized: uptake, missed pills, side effects, discontinuation and switching, drug interactions and monitoring. Additional evidence and experts were consulted to answer outstanding questions and counseling recommendations for the DPP were developed. Missed pills was the topic with the most complexity, raising questions about whether women could "double up" on missed pills or skip the last week of the pack to recover protection faster. Uptake required aligning the time to reach protective levels for both DPP components and explaining the need to take DPP pills during week 4 of the pack. The potential intensity of DPP side effects, given the combination of oral PrEP with COC, was an important consideration. Discontinuation and switching looked at managing risk of HIV and unintended pregnancy when stopping or switching from the DPP. Guidance on drug interactions contended with differing contraindications for COC and PrEP. Monitoring required balancing clinical requirements with potential user burden.
Actionable recommendations: The working group developed counseling recommendations for the DPP to be tested in clinical acceptability studies. Uptake: Take one pill every day for the DPP until the pack is empty. Days 1-21 contain COC and oral PrEP. Days 22-28 do not contain COC to allow for monthly bleeding, but do contain oral PrEP and pills should be taken to maintain HIV protection. Take the DPP for 7 consecutive days to reach protective levels against pregnancy and HIV. Missed pills: If you miss 1 pill multiple times in a month or 2+ consecutive pills, take the DPP as soon as you remember. Do not take more than 2 pills in a day. If 2+ consecutive pills are missed, only take the last missed pill and discard the other missed pills. Side effects: You may experience side effects when you start using the DPP, including changes to monthly bleeding. Side effects are typically mild and go away without treatment. Discontinuation/switching: If you decide to discontinue use of the DPP, but want to be protected from HIV and/or unintended pregnancy, in most cases, you can begin using PrEP or another contraceptive method right away. Drug interactions: There are no drug-drug interactions from combining oral PrEP and COC in the DPP. Certain medications are not recommended due to their contraindication with oral PrEP or COC. Monitoring: You will need to get an HIV test prior to initiating or restarting the DPP, and every 3 months during DPP use. Your provider may recommend other screening or testing.
Discussion: Developing recommendations for the DPP as a novel MPT posed unique challenges, with implications for efficacy, cost, and user and provider comprehension and burden. Incorporating counseling recommendations into clinical cross-over acceptability studies allows for real-time feedback from providers and users. Supporting women with information to use the DPP correctly and confidently is critically important for eventual scale and commercialization.
Keywords: HIV prevention; family planning; multi-purpose prevention technologies; oral contraception; pre-exposure prophlyaxis; provider counseling; service delivery; sexual and reproductive health.
© 2023 Segal, Harris, Carmone, Haddad, Hadigal, Hatzold, Jones, Lathrop, Mason and Mikulich.
Conflict of interest statement
SH is employed by Viatris, a developer of a DPP. LBH is employed by the Population Council, a non-profit developer of reproductive health products, including a different DPP. The work described in this paper does not specifically pertain to research and development of a DPP product. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
"Killing two birds with one stone" - a qualitative study on women's perspectives on the dual prevention pill in Johannesburg, South Africa.BMC Womens Health. 2024 Aug 22;24(1):462. doi: 10.1186/s12905-024-03269-8. BMC Womens Health. 2024. PMID: 39174929 Free PMC article.
-
Cost-effectiveness of the dual prevention pill for contraception and HIV pre-exposure prophylaxis.Front Reprod Health. 2023 May 17;5:1144217. doi: 10.3389/frph.2023.1144217. eCollection 2023. Front Reprod Health. 2023. PMID: 37266447 Free PMC article.
-
The Promise of the Dual Prevention Pill: A Framework for Development and Introduction.Front Reprod Health. 2021 Jun;3:682689. doi: 10.3389/frph.2021.682689. Epub 2021 Jun 23. Front Reprod Health. 2021. PMID: 34318291 Free PMC article.
-
Combined oral contraceptives: acceptability and effective use.Br Med Bull. 1993 Jan;49(1):140-57. doi: 10.1093/oxfordjournals.bmb.a072593. Br Med Bull. 1993. PMID: 8324604 Review.
-
Canadian Contraception Consensus (Part 1 of 4).J Obstet Gynaecol Can. 2015 Oct;37(10):936-42. doi: 10.1016/s1701-2163(16)30033-0. J Obstet Gynaecol Can. 2015. PMID: 26606712 English, French.
Cited by
-
Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape.J Int AIDS Soc. 2024 Aug;27(8):e26346. doi: 10.1002/jia2.26346. J Int AIDS Soc. 2024. PMID: 39148275 Free PMC article.
-
Assessing the acceptability of, adherence to and preference for a dual prevention pill (DPP) for HIV and pregnancy prevention compared to oral pre-exposure prophylaxis (PrEP) and oral contraception taken separately: protocols for two randomised, controlled, cross-over studies in South Africa and Zimbabwe.BMJ Open. 2024 Mar 12;14(3):e075381. doi: 10.1136/bmjopen-2023-075381. BMJ Open. 2024. PMID: 38479746 Free PMC article.
-
Reversible female contraceptives: historical, current, and future perspectives†.Biol Reprod. 2024 Jan 13;110(1):14-32. doi: 10.1093/biolre/ioad154. Biol Reprod. 2024. PMID: 37941453 Free PMC article. Review.
-
"…this could be a noble idea and a game changer." The potential of a dual prevention pill for HIV and pregnancy prevention among adolescent girls and young women in Zimbabwe.PLOS Glob Public Health. 2025 Aug 28;5(8):e0005071. doi: 10.1371/journal.pgph.0005071. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 40875750 Free PMC article.
References
-
- UNAIDS. AIDSinfo. Available at: https://aidsinfo.unaids.org/ (Accessed December 21, 2022).
-
- United Nations Population Fund (UNFPA). State of the world population 2022. New York: UNFPA; (2022).
-
- Haakenstad A, Angelino O, Irvine CMS, Bhutta ZA, Bienhoff K, Bintz C, et al. Measuring contraceptive method mix, prevalence, and demand satisfied by age and marital status in 204 countries and territories, 1970–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2022) 400:295–327. 10.1016/S0140-6736(22)00936-9 - DOI - PMC - PubMed
-
- Roberts ST, Haberer J, Celum C, Mugo N, Ware NC, Cohen CR, et al. Intimate partner violence and adherence to HIV pre-exposure prophylaxis (PrEP) in African women in HIV serodiscordant relationships: a prospective cohort study. J Acquir Immune Defic Syndr. (2016) 73(3):313–22. 10.1097/QAI.0000000000001093 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous