Adjuvant denosumab in early breast cancer: a systematic review and meta-analysis of randomized controlled clinical trials
- PMID: 37284523
- PMCID: PMC10240867
- DOI: 10.1177/17588359231173180
Adjuvant denosumab in early breast cancer: a systematic review and meta-analysis of randomized controlled clinical trials
Abstract
Background: In early breast cancer (BC) the impact of denosumab on survival outcomes is still unclear. We undertook a systematic review and meta-analysis to assess efficacy and safety of adjuvant denosumab in addition to standard anticancer therapy.
Methods: PubMed, CENTRAL, Scopus, Embase, and oncological meetings websites were screened to identify potentially eligible randomized controlled trials (RCTs). Survival outcomes were disease-free survival (DFS), bone-metastasis-free survival (BMFS), and overall survival (OS). Fracture incidence and time to first fracture were bone-health outcomes. Osteonecrosis of the jaw (ONJ), atypical femur fractures (AFF), and other adverse events were also evaluated. Pooled hazard ratios (HRs) and risk ratios (RR) with respective 95% confidence interval (95% CI) were computed using a random-effects model. Exploratory subgroup analyses were performed.
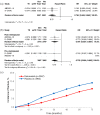
Results: Two phase III RCTs were included, the Austrian Breast & Colorectal Cancer Study Group-18 (ABCSG-18) and the D-CARE trials, for a total of 7929 patients. In the ABCSG-18 trial, denosumab was administered every 6 months during endocrine therapy (for a median of seven cycles) while the D-CARE trial used an intensive schedule for a total treatment duration of 5 years. Adjuvant denosumab showed no difference in DFS (HR: 0.932; 95% CI: 0.748-1.162), BMFS (HR: 0.9896; 95% CI: 0.751-1.070), and OS (HR: 0.917; 95% CI: 0.718-1.171) compared to placebo in the overall population. In hormone receptor positive/human epidermal growth factor receptor 2 (HER2) negative BC patients, a DFS (HR: 0.883; 95% CI: 0.782-0.996) and BMFS (HR: 0.832; 95% CI: 0.714-0.970) benefit was observed and BMFS was prolonged in all hormone receptor positive patients (HR: 0.850; 95% CI: 0.735-0.983). Fracture incidence (RR: 0.787; 95% CI: 0.696-0.890) and time to first fracture (HR: 0.760; 95% CI: 0.665-0.869) were also improved. No increase in overall toxicity was seen with denosumab and no differences were observed for ONJ and AFF between the 60-mg every 6-month schedule and placebo.
Conclusion: Denosumab addition to anticancer treatment does not improve DFS, BMFS, or OS in the overall population, although a DFS improvement was observed in hormone receptor positive/HER2 negative BC patients and a BMFS improvement in all hormone receptor positive patients. Bone-health outcomes were improved with no added toxicity with the 60-mg schedule.
Registration: PROSPERO identifier: CRD42022332787.
Keywords: adjuvant therapy; bone-health; denosumab; early breast cancer; meta-analysis.
© The Author(s), 2023.
Conflict of interest statement
LM, GG, EDM, NM, MP, LP, SP, MCC, and ADB declare no conflict of interest. AP has declared consulting fees/advisory role for Amgen, MSD, Novartis, travel and accommodation by Pfizer. AF has declared consulting fees/advisory role for AstraZeneca, Daiichi Sankyo, Eisai, Eli-Lilly. Epionpharma, exact science, MSD, Novartis, Pierre Fabre, Roche, Seagen. GT is supported by funds of Ministero della Salute (Ricerca Corrente 2022). EB is currently supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under Investigator Grant (IG) No. IG20583. EB is supported by institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1). EB is supported by funds of Ministero della Salute (Ricerca Corrente 2022). EB received speakers’ and travels’ fee from MSD, AstraZeneca, Pfizer, Eli-Lilly, BMS, Novartis, and Roche. EB received institutional research grants from AstraZeneca, Roche. AO has declared consulting fees/advisory role for Novartis, Roche, Eli-Lilly, Amgen, Daiichi Sankyo and travel and accommodation by Daiichi Sankyo, Novartis, Roche, and Pfizer.
Figures







References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. - PubMed
-
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1194–1220. - PubMed
-
- Bradley R, Braybrooke J, Gray R, et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol 2022; 23: 382–392. - PMC - PubMed
-
- Bouvard B, Soulié P, Hoppé E, et al. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann Oncol 2014; 25: 843–847. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

