Exoproducts of the Most Common Achromobacter Species in Cystic Fibrosis Evoke Similar Inflammatory Responses In Vitro
- PMID: 37284754
- PMCID: PMC10434066
- DOI: 10.1128/spectrum.00195-23
Exoproducts of the Most Common Achromobacter Species in Cystic Fibrosis Evoke Similar Inflammatory Responses In Vitro
Abstract
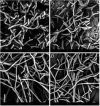
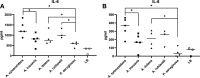
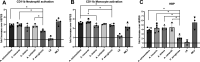
Achromobacter is a genus of Gram-negative rods, which can cause persistent airway infections in people with cystic fibrosis (CF). The knowledge about virulence and clinical implications of Achromobacter is still limited, and it is not fully established whether Achromobacter infections contribute to disease progression or if it is a marker of poor lung function. The most commonly reported Achromobacter species in CF is A. xylosoxidans. While other Achromobacter spp. are also identified in CF airways, the currently used Matrix-Assisted Laser Desorption/Ionization Time Of Flight Mass Spectrometry (MALDI-TOF MS) method in routine diagnostics cannot distinguish between species. Differences in virulence between Achromobacter species have consequently not been well studied. In this study, we compare phenotypes and proinflammatory properties of A. xylosoxidans, A. dolens, A. insuavis, and A. ruhlandii using in vitro models. Bacterial supernatants were used to stimulate CF bronchial epithelial cells and whole blood from healthy individuals. Supernatants from the well-characterized CF-pathogen Pseudomonas aeruginosa were included for comparison. Inflammatory mediators were analyzed with ELISA and leukocyte activation was assessed using flow cytometry. The four Achromobacter species differed in morphology seen in scanning electron microscopy (SEM), but there were no observed differences in swimming motility or biofilm formation. Exoproducts from all Achromobacter species except A. insuavis caused significant IL-6 and IL-8 secretion from CF lung epithelium. The cytokine release was equivalent or stronger than the response induced by P. aeruginosa. All Achromobacter species activated neutrophils and monocytes ex vivo in a lipopolysaccharide (LPS)-independent manner. Our results indicate that exoproducts of the four included Achromobacter species do not differ consistently in causing inflammatory responses, but they are equally or even more capable of inducing inflammation compared with the classical CF pathogen P. aeruginosa. IMPORTANCE Achromobacter xylosoxidans is an emerging pathogen among people with cystic fibrosis (CF). Current routine diagnostic methods are often unable to distinguish A. xylosoxidans from other Achromobacter species, and the clinical relevance of different species is still unknown. In this work, we show that four different Achromobacter species relevant to CF evoke similar inflammatory responses from airway epithelium and leukocytes in vitro, but they are all equally or even more proinflammatory compared to the classic CF-pathogen Pseudomonas aeruginosa. The results suggest that Achromobacter species are important airway pathogens in CF, and that all Achromobacter species are relevant to treat.
Keywords: Achromobacter dolens; Achromobacter insuavis; Achromobacter ruhlandii; Achromobacter xylosoxidans; Pseudomonas aeruginosa; cystic fibrosis; in vitro inflammatory responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

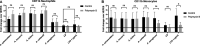
References
-
- Schupp JC, Khanal S, Gomez JL, Sauler M, Adams TS, Chupp GL, Yan X, Poli S, Zhao Y, Montgomery RR, Rosas IO, Dela Cruz CS, Bruscia EM, Egan ME, Kaminski N, Britto CJ. 2020. Single-cell transcriptional archetypes of airway inflammation in cystic fibrosis. Am J Respir Crit Care Med 202:1419–1429. doi:10.1164/rccm.202004-0991OC. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

