Isolation and characterization of a VHH targeting the Acinetobacter baumannii cell surface protein CsuA/B
- PMID: 37284893
- PMCID: PMC10246537
- DOI: 10.1007/s00253-023-12594-1
Isolation and characterization of a VHH targeting the Acinetobacter baumannii cell surface protein CsuA/B
Abstract
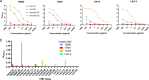
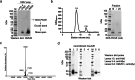
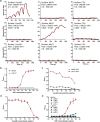
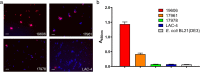
Acinetobacter baumannii is a Gram-negative bacterial pathogen that exhibits high intrinsic resistance to antimicrobials, with treatment often requiring the use of last-resort antibiotics. Antibiotic-resistant strains have become increasingly prevalent, underscoring a need for new therapeutic interventions. The aim of this study was to use A. baumannii outer membrane vesicles as immunogens to generate single-domain antibodies (VHHs) against bacterial cell surface targets. Llama immunization with the outer membrane vesicle preparations from four A. baumannii strains (ATCC 19606, ATCC 17961, ATCC 17975, and LAC-4) elicited a strong heavy-chain IgG response, and VHHs were selected against cell surface and/or extracellular targets. For one VHH, OMV81, the target antigen was identified using a combination of gel electrophoresis, mass spectrometry, and binding studies. Using these techniques, OMV81 was shown to specifically recognize CsuA/B, a protein subunit of the Csu pilus, with an equilibrium dissociation constant of 17 nM. OMV81 specifically bound to intact A. baumannii cells, highlighting its potential use as a targeting agent. We anticipate the ability to generate antigen-specific antibodies against cell surface A. baumannii targets could provide tools for further study and treatment of this pathogen. KEY POINTS: •Llama immunization with bacterial OMV preparations for VHH generation •A. baumannii CsuA/B, a pilus subunit, identified by mass spectrometry as VHH target •High-affinity and specific VHH binding to CsuA/B and A. baumannii cells.
Keywords: Acinetobacter baumannii; Antimicrobial resistance; CsuA/B; Nanobody; Outer membrane vesicle; Pilus; Single-domain antibody; VHH.
© 2023. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Baral TN, MacKenzie R, Arbabi Ghahroudi M. Single-domain antibodies and their utility. Curr Protoc Immunol. 2013;103:2.17.1–2.17.57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

