Radiosurgery for ventricular tachycardia (RAVENTA): interim analysis of a multicenter multiplatform feasibility trial
- PMID: 37285038
- PMCID: PMC10245341
- DOI: 10.1007/s00066-023-02091-9
Radiosurgery for ventricular tachycardia (RAVENTA): interim analysis of a multicenter multiplatform feasibility trial
Abstract
Background: Single-session cardiac stereotactic radiation therapy (SBRT) has demonstrated promising results for patients with refractory ventricular tachycardia (VT). However, the full safety profile of this novel treatment remains unknown and very limited data from prospective clinical multicenter trials are available.
Methods: The prospective multicenter multiplatform RAVENTA (radiosurgery for ventricular tachycardia) study assesses high-precision image-guided cardiac SBRT with 25 Gy delivered to the VT substrate determined by high-definition endocardial and/or epicardial electrophysiological mapping in patients with refractory VT ineligible for catheter ablation and an implanted cardioverter defibrillator (ICD). Primary endpoint is the feasibility of full-dose application and procedural safety (defined as an incidence of serious [grade ≥ 3] treatment-related complications ≤ 5% within 30 days after therapy). Secondary endpoints comprise VT burden, ICD interventions, treatment-related toxicity, and quality of life. We present the results of a protocol-defined interim analysis.
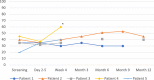
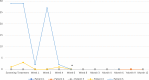
Results: Between 10/2019 and 12/2021, a total of five patients were included at three university medical centers. In all cases, the treatment was carried out without complications. There were no serious potentially treatment-related adverse events and no deterioration of left ventricular ejection fraction upon echocardiography. Three patients had a decrease in VT episodes during follow-up. One patient underwent subsequent catheter ablation for a new VT with different morphology. One patient with local VT recurrence died 6 weeks after treatment in cardiogenic shock.
Conclusion: The interim analysis of the RAVENTA trial demonstrates early initial feasibility of this new treatment without serious complications within 30 days after treatment in five patients. Recruitment will continue as planned and the study has been expanded to further university medical centers.
Trial registration number: NCT03867747 (clinicaltrials.gov). Registered March 8, 2019. Study start: October 1, 2019.
Keywords: Cardiac arrhythmia; Implantable cardioverter defibrillator; Stereotactic arrythmia radioablation; Stereotactic body radiotherapy; Structural heart disease.
© 2023. The Author(s).
Conflict of interest statement
D. Buergy reports personal fees from Siemens AG, NB Capital Research GmbH, NB Capital ApS, b.e. Imaging GmbH, and PharmaMar, all outside the submitted work. D. Krug has received honoraria from MSD Sharp & Dohme and Pfizer as well as research funding from Merck KGaA, outside the submitted work. B. Rudic received travel grants and personal fees from Boston Scientific Medizintechnik GmbH, outside of the submitted work. J. Boda-Heggemann reports personal fees from EBAMed SA, outside the submitted work. S. Hohmann received travel grants from Boston Scientific Medizintechnik GmbH, outside of the submitted work. L.-H. Boldt received honoraria from Biotronik, Boston Scientific, and Pfizer, outside of the submitted work. O. Blanck is the section editor for medical physics of the
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

