Hydrogel-Encapsulated Biofilm Inhibitors Abrogate the Cariogenic Activity of Streptococcus mutans
- PMID: 37285134
- PMCID: PMC11188996
- DOI: 10.1021/acs.jmedchem.3c00272
Hydrogel-Encapsulated Biofilm Inhibitors Abrogate the Cariogenic Activity of Streptococcus mutans
Abstract
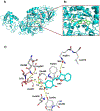
We designed and synthesized analogues of a previously identified biofilm inhibitor IIIC5 to improve solubility, retain inhibitory activities, and to facilitate encapsulation into pH-responsive hydrogel microparticles. The optimized lead compound HA5 showed improved solubility of 120.09 μg/mL, inhibited Streptococcus mutans biofilm with an IC50 value of 6.42 μM, and did not affect the growth of oral commensal species up to a 15-fold higher concentration. The cocrystal structure of HA5 with GtfB catalytic domain determined at 2.35 Å resolution revealed its active site interactions. The ability of HA5 to inhibit S. mutans Gtfs and to reduce glucan production has been demonstrated. The hydrogel-encapsulated biofilm inhibitor (HEBI), generated by encapsulating HA5 in hydrogel, selectively inhibited S. mutans biofilms like HA5. Treatment of S. mutans-infected rats with HA5 or HEBI resulted in a significant reduction in buccal, sulcal, and proximal dental caries compared to untreated, infected rats.
Figures
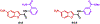
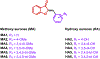



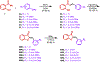
References
-
- Marsh PD Dental Plaque as a Microbial Biofilm. Caries Res. 2004, 38, 204–211. - PubMed
-
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ, Coggeshall M, Cornaby L, Dandona L, Dicker DJ, Dilegge T, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming T, … Murray CJL. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. - PMC - PubMed
-
- Overman PR Biofilm: A New View of Plaque. J. Contemp. Dent. Pract 2000, 1, 18–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

