Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England
- PMID: 37285378
- PMCID: PMC10286992
- DOI: 10.1371/journal.pmed.1004245
Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England
Abstract
Background: An increased risk of myocarditis or pericarditis after priming with mRNA Coronavirus Disease 2019 (COVID-19) vaccines has been shown but information on the risk post-booster is limited. With the now high prevalence of prior Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, we assessed the effect of prior infection on the vaccine risk and the risk from COVID-19 reinfection.
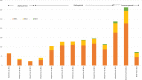
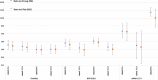
Methods and findings: We conducted a self-controlled case series analysis of hospital admissions for myocarditis or pericarditis in England between 22 February 2021 and 6 February 2022 in the 50 million individuals eligible to receive the adenovirus-vectored vaccine (ChAdOx1-S) for priming or an mRNA vaccine (BNT162b2 or mRNA-1273) for priming or boosting. Myocarditis and pericarditis admissions were extracted from the Secondary Uses Service (SUS) database in England and vaccination histories from the National Immunisation Management System (NIMS); prior infections were obtained from the UK Health Security Agency's Second-Generation Surveillance Systems. The relative incidence (RI) of admission within 0 to 6 and 7 to 14 days of vaccination compared with periods outside these risk windows stratified by age, dose, and prior SARS-CoV-2 infection for individuals aged 12 to 101 years was estimated. The RI within 27 days of an infection was assessed in the same model. There were 2,284 admissions for myocarditis and 1,651 for pericarditis in the study period. Elevated RIs were only observed in 16- to 39-year-olds 0 to 6 days postvaccination, mainly in males for myocarditis. Both mRNA vaccines showed elevated RIs after first, second, and third doses with the highest RIs after a second dose 5.34 (95% confidence interval (CI) [3.81, 7.48]; p < 0.001) for BNT162b2 and 56.48 (95% CI [33.95, 93.97]; p < 0.001) for mRNA-1273 compared with 4.38 (95% CI [2.59, 7.38]; p < 0.001) and 7.88 (95% CI [4.02, 15.44]; p < 0.001), respectively, after a third dose. For ChAdOx1-S, an elevated RI was only observed after a first dose, RI 5.23 (95% CI [2.48, 11.01]; p < 0.001). An elevated risk of admission for pericarditis was only observed 0 to 6 days after a second dose of mRNA-1273 vaccine in 16 to 39 year olds, RI 4.84 (95% CI [1.62, 14.01]; p = 0.004). RIs were lower in those with a prior SARS-CoV-2 infection than in those without, 2.47 (95% CI [1.32,4.63]; p = 0.005) versus 4.45 (95% [3.12, 6.34]; p = 0.001) after a second BNT162b2 dose, and 19.07 (95% CI [8.62, 42.19]; p < 0.001) versus 37.2 (95% CI [22.18, 62.38]; p < 0.001) for mRNA-1273 (myocarditis and pericarditis outcomes combined). RIs 1 to 27 days postinfection were elevated in all ages and were marginally lower for breakthrough infections, 2.33 (95% CI [1.96, 2.76]; p < 0.001) compared with 3.32 (95% CI [2.54, 4.33]; p < 0.001) in vaccine-naïve individuals respectively.
Conclusions: We observed an increased risk of myocarditis within the first week after priming and booster doses of mRNA vaccines, predominantly in males under 40 years with the highest risks after a second dose. The risk difference between the second and the third doses was particularly marked for the mRNA-1273 vaccine that contains half the amount of mRNA when used for boosting than priming. The lower risk in those with prior SARS-CoV-2 infection, and lack of an enhanced effect post-booster, does not suggest a spike-directed immune mechanism. Research to understand the mechanism of vaccine-associated myocarditis and to document the risk with bivalent mRNA vaccines is warranted.
Copyright: © 2023 Stowe et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
-
A Systematic Review and Meta-analysis of the Association Between SARS-CoV-2 Vaccination and Myocarditis or Pericarditis.Am J Prev Med. 2023 Feb;64(2):275-284. doi: 10.1016/j.amepre.2022.09.002. Epub 2022 Sep 26. Am J Prev Med. 2023. PMID: 36266115 Free PMC article.
-
Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series.Rev Med Virol. 2022 Jul;32(4):e2318. doi: 10.1002/rmv.2318. Epub 2021 Dec 17. Rev Med Virol. 2022. PMID: 34921468
Cited by
-
Applying two approaches to detect unmeasured confounding due to time-varying variables in a self-controlled risk interval design evaluating COVID-19 vaccine safety signals, using myocarditis as a case example.Am J Epidemiol. 2025 Jan 8;194(1):208-219. doi: 10.1093/aje/kwae172. Am J Epidemiol. 2025. PMID: 38960670 Free PMC article.
-
How do large-scale population studies inform vaccine evaluations in England?Clin Exp Immunol. 2025 Jan 21;219(1):uxaf006. doi: 10.1093/cei/uxaf006. Clin Exp Immunol. 2025. PMID: 39910973 Review.
-
Myocarditis associated with COVID-19 vaccination.NPJ Vaccines. 2024 Jun 28;9(1):122. doi: 10.1038/s41541-024-00893-1. NPJ Vaccines. 2024. PMID: 38942751 Free PMC article. Review.
-
Vaccine Hesitancy and Associated Factors Amongst Health Professionals: A Scoping Review of the Published Literature.Vaccines (Basel). 2024 Dec 13;12(12):1411. doi: 10.3390/vaccines12121411. Vaccines (Basel). 2024. PMID: 39772072 Free PMC article.
-
The risk of acute disseminated encephalomyelitis (ADEM) following covid-19 vaccination in England: A self-controlled case-series analysis.Hum Vaccin Immunother. 2024 Dec 31;20(1):2311969. doi: 10.1080/21645515.2024.2311969. Epub 2024 Feb 1. Hum Vaccin Immunother. 2024. PMID: 38299507 Free PMC article.
References
-
- Thompson MG. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022. [cited 2022 Jan 26];71. Available from: https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e3.htm (accessed 2023 Apr 24). - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

